......

Posaconazole 泊沙康唑 , بوساكونازول , Позаконазол
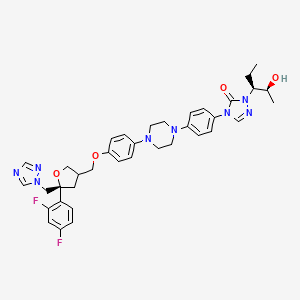
4-[4-[4-[4-[[(5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)oxolan-3-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-hydroxypentan-3-yl]-1,2,4-triazol-3-one
................
http://www.google.com/patents/WO2013042138A2?cl=en
HPLC Method of Analysis for Posaconazole:
Posaconazole is analyzed by HPLC using the following conditions: Apparatus: A liquid chromatographic system is to be equipped with variable wavelength UV-detector; Column: Grace Alltima CI 8, 150 x 4.6mm 3μηι or equivalent; Flow rate: 1.0 ml/min; Wavelength: 210 nm; Column Temperature: 28°C; Injection volume: 10
Run time: 60 min; Diluent: Acetonitrile: water (50:50 v/v); Needle wash: Acetonitrile: water (50:50 v/v); Elution: Gradient; Mobile phase-A: Buffer Acetonitrile (90: 10) v/v; Mobile phase- B: Acetonitrile: water (90:10) v/v; Buffer: 1.74 grams of potassium hydrogen phosphate in 1000 ml of water. Adjust pH to 6.5 with diluted orthophosphoric acid and filtered through 0.45μηι Nylon membrane filter paper and sonicate to degas it. PSD method of analysis for Posaconazole:
The particle size distribution of posaconazole compound of formual-1 is measured using the following conditions:
Instrument: Malvern Master sizer 2000; Measuring range: 0.02 to 2000 μπι; Wet sample: Hydro 2000S; Dispersant: Water; Absorption Index: 0; Refractive Index of water: 1.330; Refractive Index of particle: 1.500; Stirrer speed: 2500 rpm; Obscuration range: 10-20%; Sensitivity: Normal; Measurement time: 12 seconds; Background time: 12 seconds; Internal sonication: 3 minutes; (Tip displacement-70%); Measurement repeat: 3 times at zero second interval.
HPLC Method of Analysis for Benzylated Posaconazole:
Benzylated posaconazole is analyzed by HPLC using the following conditions:
Apparatus: A liquid chromatographic system is to be equipped with variable wavelength UV-detector; Column: X-bridge C18, 50X 4.6mm, 3.5um (or) equivalent; Flow rate: 0.8 ml/min; Wavelength: 210 nm; Column Temperature: 40°C; Injection volume: 5 μΐ,; Run time: 35 min; Diluent: Water: Acetonitrile (40:60) v/v; Needle wash: Water: Acetonitrile (40:60) v/v; Elution: Gradient; Mobile phase-A: Buffer Acetonitrile (90: 10) v/v; Mobile phase-B: Acetonitrile: water (90:10) v/v; Buffer: 1.74 grams of potassium hydrogen phosphate dibasic (anhydrous) in 1000 ml of Milli-Q- Water. Adjust its pH to 6.5 with diluted orthophosphoric acid and filtered through 0.22μπι Nylon membrane filter paper and sonicate to degas it. PXRD analysis of crystalline triazole antifungal compound of formula- 1 was carried out using BRUKER/AXS X-Ray diffractometer using Cu Ka radiation of wavelength 1.5406 A° and continuous scan speed of 0.03°/min.
RS/OVI analysis of amorphous posaconazole is carried out on Agilent GC-6850 series-2 with Flame Ionization detector, column AP vac, flow 2 psi and load is 1 μΐ, detector temperature is 260°C and carrier gas is helium.
The process of the present invention is schematically represented as below:
Scheme-I
ormiia- ormua-
Scheme-II:
Formula-16 Scheme-Ill:
HO N N- NH,
Formula-18
Pure POSACONAZOLE
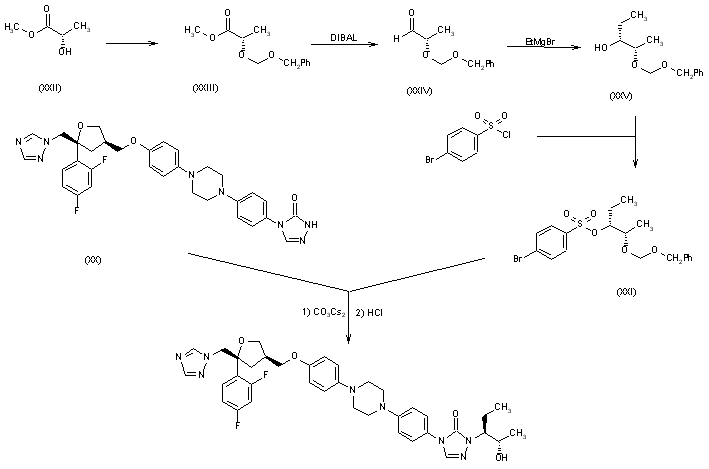
The bromosulfonate (XXI) has been obtained as follows: (S)-Lactic acid methyl ester (XXII) has been protected as its benzyloxymethyl ether (XXIII) according to Tetrahedron Lett 1980, 21: 1035. The reduction of (XXIII) with DIBAL yields the corresponding aldehyde (XXIV), which by a Grignard reaction with ethylmagnesium bromide in THF and chromatographic separation of the diastereoisomers (SiO2, hexane/ethyl acetate) affords 2(S)-(benzyloxymethoxy)-3(R)-pentanol (XXV). Finally, this compound is sulfonated to (XXI) with 4-bromobenzenesulfonyl chloride. Finally, compound (XX) is condensed with 2(S)-(benzyloxymethoxy)-3(R)-pentanol 4-bromobenzenesulfonate ester (XXI) by means of cesium carbonate in DMF, and deprotected with 6N HCl.
.....................
WO 9633178

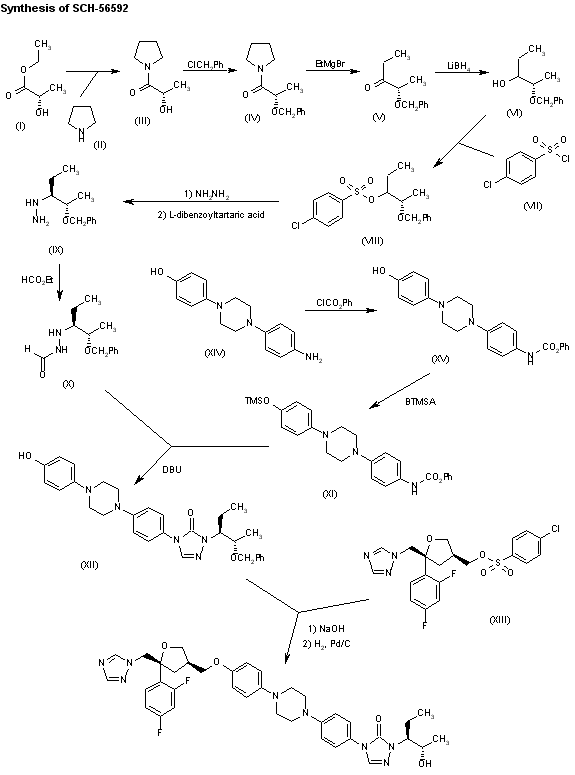
A new synthesis of Sch-56592 has been described: The reaction of (S)-ethyl lactate (I) with pyrrolidine (II) gives 1-[(S)-lactoyl]pyrrolidine (III), which is benzylated as usual with benzyl chloride yielding the benzyl ether (IV). The reaction of (IV) with ethylmagnesium bromide in THF affords 2(S)-benzyloxy-3-pentanone (V), which is reduced with LiBH4 in dimethoxyethane giving 2(S)-benzyloxy-3(RS)-pentanol (VI). The reaction of (VI) with 4-chlorobenzenesulfonyl chloride (VII) yields the corresponding sulfonate (VIII), which is treated with hydrazine in ethanol to afford a diastereomeric mixture of hydrazines that is resolved with L-dibenzoyltartaric acid giving the (S,S)-enantiomer (IX). The formylation of (IX) with refluxing ethyl formate yields the chiral formyl hydrazide (X), which is cyclized with N-[4-[4-[4-(trimethylsilyloxy)phenyl]piperazin-1-yl]phenyl]carbamic acid phenyl ester (XI) affording the triazolone (XII). Finally, this compound is condensed with the chiral tetrahydrofuran derivative (XIII) by means of NaOH in DMSO, and debenzylated by hydrogenation with H2 over Pd/C in formic acid
.....................
\35th Intersci Conf Antimicrob Agents Chemother (Sept 17-20, San Francisco) 1995,Abst F83.
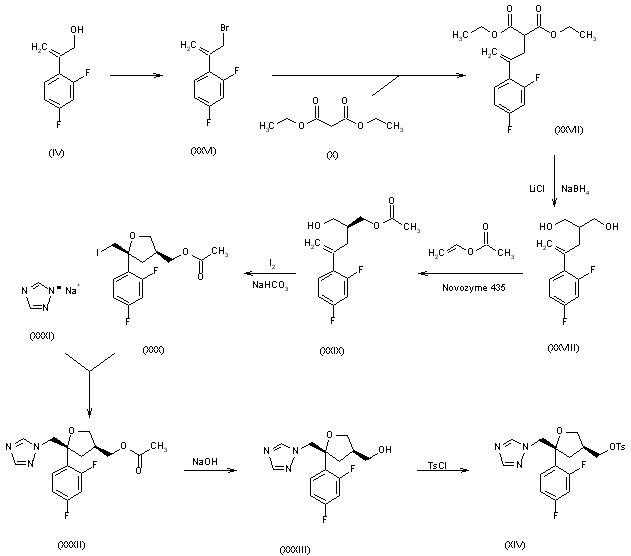
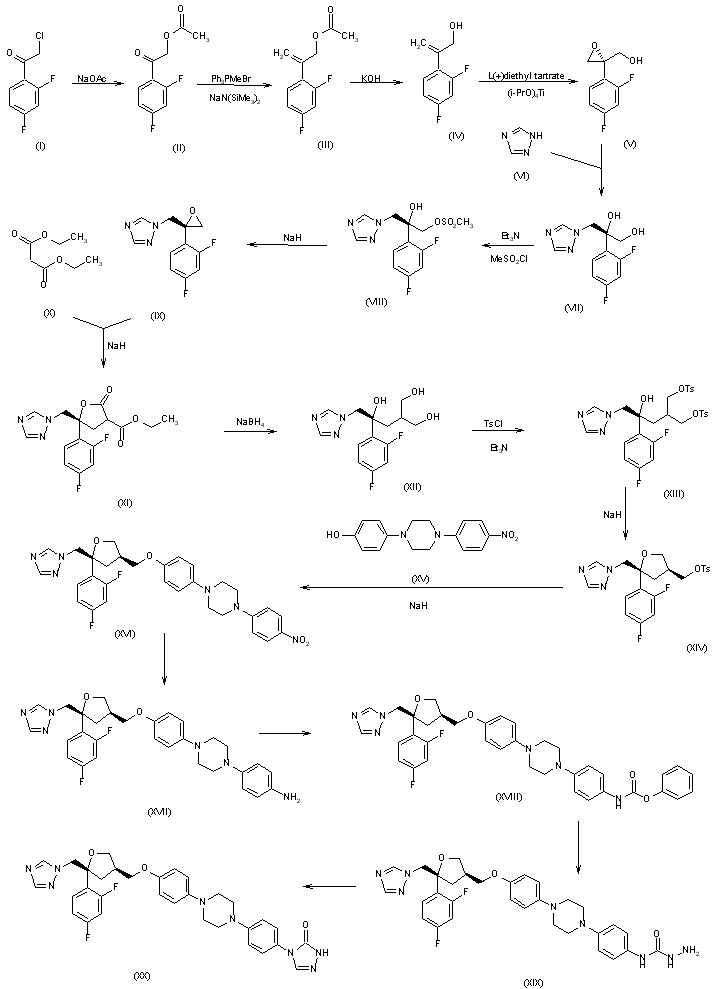
2) The 2-(2,4-difluorophenyl)-2-propen-1-ol (IV) is converted into the corresponding propenyl bromide (XXVI), which is condensed with diethyl malonate (X) to afford the malonyl derivative (XXVII). The reduction of (XXVII) with NaBH4 and LiCl yields the 1,3-propanediol derivative (XXVIII), which is enantioselectively acetylated with vinyl acetate and Novozyme 435 in acetonitrile yielding the isomeric (S)-monoacetate (XXIX). The cyclization of (XXIX) with iodine and NaHCO3 in acetonitrile affords (5R-cis)-5-(2,4-difluorophenyl)-5-(iodomethyl)tetrahydrofuran-3-methanol acetate ester (XXX), which is condensed with sodium 1,2,4-triazole (XXXI) to give (5R-cis)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl) tetrahydrofuran-3-methanol acetate ester (XXXII). The hydrolysis of (XXXII) with NaOH yields the corresponding methanol (XXXIII), which is finally tosylated to the tosyl ester (XIV), already obtained previously.
.........................
35th Intersci Conf Antimicrob Agents Chemother (Sept 17-20, San Francisco) 1995,Abst F61
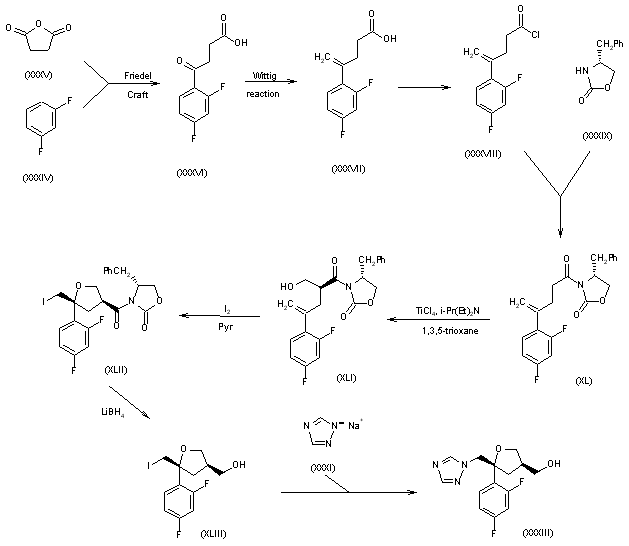
3) The Friedel Crafts condensation of m-difluorobenzene (XXXIV) with succinic anhydride (XXXV) gives 4-(2,4-difluorophenyl)-4-oxobutyric acid (XXXVI), which is converted by a Wittig reaction into 4-(2,4-difluorophenyl)-4-pentenoic acid (XXXVII) and subsequently into its acyl chloride (XXXVIII). The condensation of (XXXVIII) with 4(R)-benzyloxazolidin-2-one (XXXIX) gives the acyl oxazolidinone (XL), which is regioselectively hydroxymethylated with 1,3,5-trioxane and TiCl4 to afford 4(R)-benzyl-3-[4-(2,4-difluorophenyl)-3(S)-(hydroxymethyl)-4-pentenoyl] oxazolidin-2-one (XLI). The cyclization of (XLI) with iodine and pyridine yields the tetrahydrofuran derivative (XLII), which is reduced with LiBH4 to (5R-cis)-5-(2,4-difluoromethyl)-5-(iodomethyl)tetrahydrofuran-3-methanol (XLIII). Finally, this compound is condensed with sodium 1,2,4-triazole (XXXI) to afford (5R-cis)-5-(2,4-difluoromethyl)-5-(1,2,4-triazol-1-ylmethyl) tetrahydrofuran-3-methanol (XXXIII), already obtained in Scheme 22656201c.
.................
J Label Compd Radiopharm 1998,41(8),731

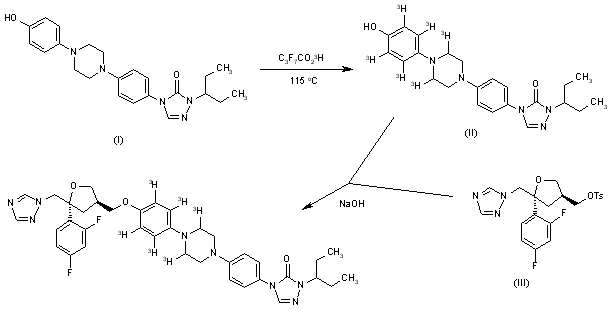
The synthesis of [3H]-SCH-51048 has been described: The tritiation of phenol (I) with tritiated heptafluorobutyric acid at 115 C gives the polytritiated intermediate (II), which is then condensed with the chiral tosylate (III) by means of NaOH in DMSO affording labeled SCH-51048.
......................
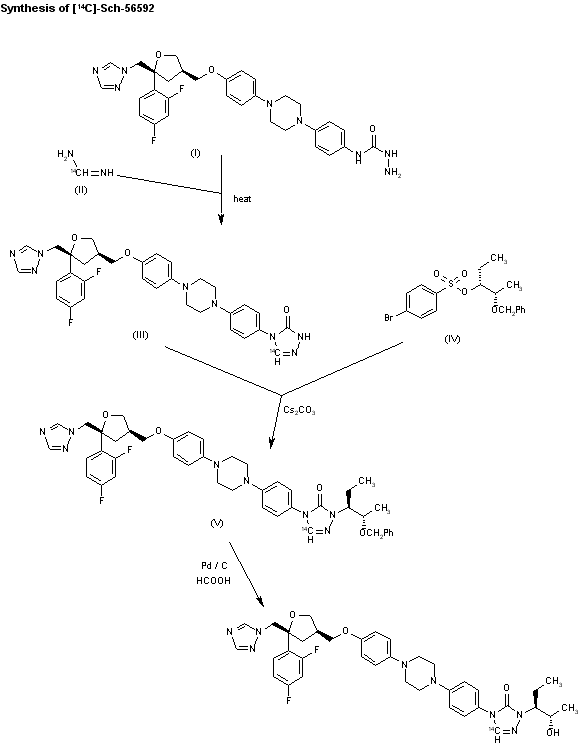
The synthesis of [14C]-SCH-56592 has been described: The cyclization of semicarbazide (I) with [14C]-formamidine (II) in hot 2-methoxyethanol gives the triazolone (III), which is condensed with the sulfonate (IV) by means of Cs2CO3 in hot DMF to yield the alkylated triazolone (V). Finally, this compound is deprotected by hydrogenation with formic acid over Pd/C in hot methanol to afford labeled SCH-56592.
..................
Tetrahedron Lett 2002,43(18),3359
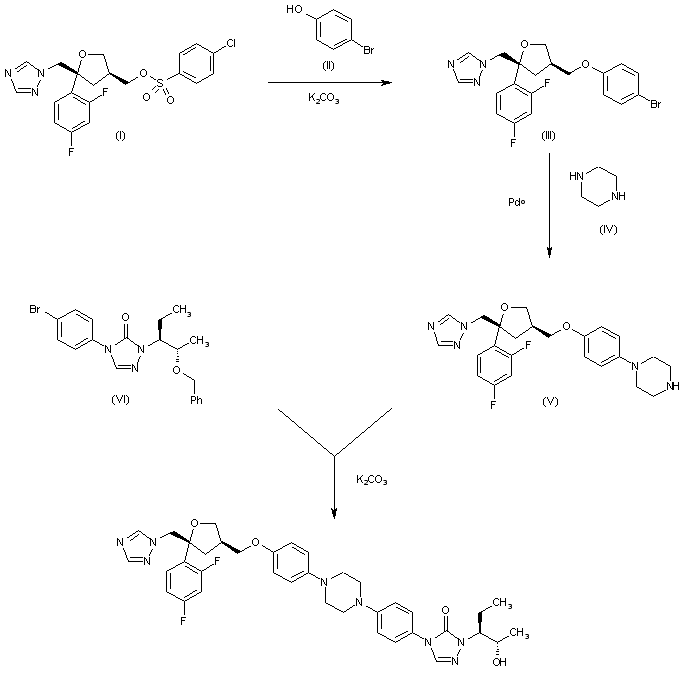
The condensation of 4-chlorophenylsulfonate (I) with 4-bromophenol (II) by means of K2CO3 in hot DMF gives the aryl ether (III), which is condensed with piperazine (IV) by means of Pdo to yield the monosubstituted piperazine (V). Finally, this compound is condensed with the 4-bromophenyltriazolone (VI) by means of K2CO3 in hot DMSO to afford the target disubstituted piperazine
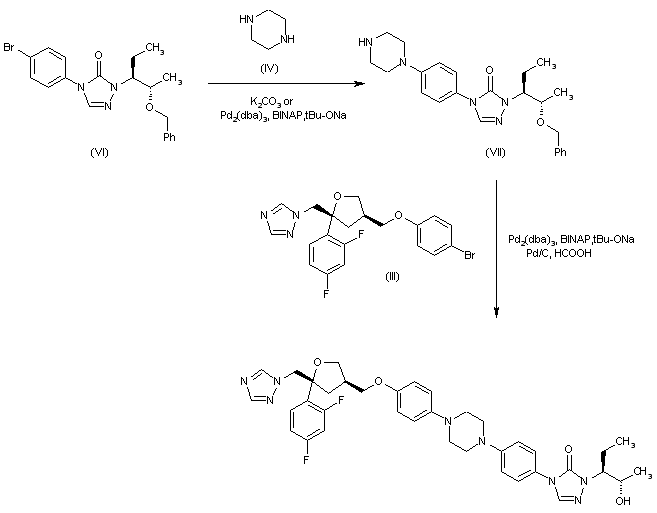
Alternatively, the condensation of with the 4-bromophenyltriazolone (VI) with piperazine (IV) by means of Pd2(dba)3, BINAP and t-BuONa in hot toluene gives the monosubstituted piperazine (VII), which is then condensed with the already reported aryl ether (III) by means of Pd2(dba)3, BINAP and t-BuONa in hot toluene, and debenzylated with Pd/C and formic acid to afford the target disubstituted piperazine.
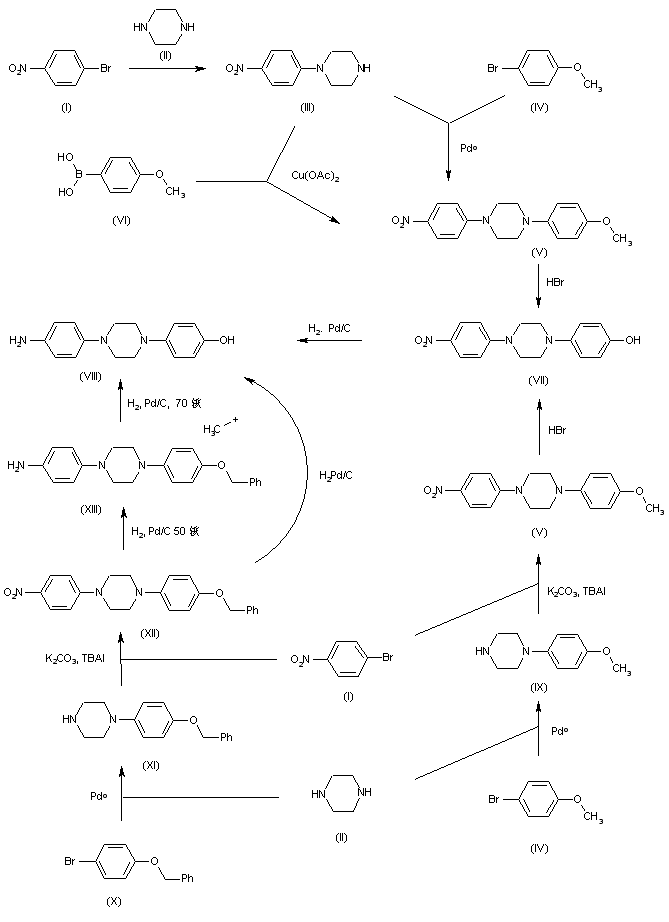
The intermediate 4-[4-(4-aminophenyl)piperazin-1-yl]phenol (VIII) has been obtained by several related ways: 1.- The condensation of 4-bromonitrobenzene (I) with piperazine (II) gives 1-(4-nitrophenyl)piperazine (III), which is condensed with 4-bromoanisole (IV) by means of Pdo to yield 1-(4-methoxyphenyl)-4-(4-nitrophenyl)piperazine (V). Alternatively, (V) can also be obtained by condensation of (III) with 4-methoxyphenylboronic acid (VI) by means of Cu(OAc)2 in DMSO. The demethylation of (V) with HBr yields 4-[4-(4-nitrophenyl)piperazin-1-yl]phenol (VII), which is finally reduced with H2 over Pd/C to afford the target 4-[4-(4-aminophenyl)piperazin-1-yl]phenol (VIII) intermediate (see Synthline, scheme no. 22656202a, intermediate (XIV)). 2.- The condensation of piperazine (II) with 4-bromoanisole (IV) by means of Pdo gives 1-(4-methoxyphenyl)piperazine (IX), which is condensed with 4-bromonitrobenzene (I) by means of K2CO3 and tetrabutylammonium iodide (TBAI) in hot DMSO to yield intermediate 1-(4-methoxyphenyl)-4-(4-nitrophenyl)piperazine (V), already reported. 3.- The condensation of piperazine (II) with 4-(benzyloxy)phenyl bromide (X) by means of Pdo gives 1-(4-benzyloxyphenyl)piperazine (XI), which is condensed with 4-bromonitrobenzene (I) by means of K2CO3 and TBAI in hot DMSO to yield 1-(4-benzyloxyphenyl)-4-(4-nitrophenyl)piperazine (XII). The nitro group of (XII) is reduced by means of H2 (50 psi) over Pd/C in wet THF at 50? C to afford 4-[4-(4-benzyloxyphenyl)piperazin-1-yl]aniline (XIII), which is finally debenzylated with H2 (80 psi) over Pd/C in wet THF at 70? C or other drastic conditions to afford the target 4-[4-(4-aminophenyl)piperazin-1-yl]phenol (VIII) intermediate (see Synthline, scheme no. 22656202a, intermediate (XIV)). Alternatively, 1-(4-benzyloxyphenyl)-4-(4-nitrophenyl)piperazine (XII) can also be reduced directly to the target intermediate (VIII) with H2 over Pd/C under a variety of drastic conditions.
Literature References:
Orally active triazole antifungal. Prepn: A. K. Saksena et al., WO 9517407; eidem, US 5661151 (1995, 1997 both to Schering); eidem, Tetrahedron Lett. 37, 5657 (1996).
Comparative antifungal spectrum: A. Cacciapuoti et al., Antimicrob. Agents Chemother. 44, 2017 (2000). Pharmacokinetics, safety and tolerability: R. Courtney et al., ibid. 47, 2788 (2003).
HPLC determn in serum: H. Kim et al., J. Chromatogr. B 738, 93 (2000).
Review of development: A. K. Saksena et al. inAnti-Infectives: Recent Advances in Chemistry and Structure Activity Relationships (Royal Soc. Chem., Cambridge, 1997) pp 180-199; and clinical efficacy in fungal infections: R. Herbrecht, Int. J. Clin. Pract. 58, 612-624 (2004).


1H NMR PREDICT
13C NMR PREDICT
COSY PREDICT
/////
सुकून उतना ही देना प्रभू, जितने से जिंदगी चल जाये। औकात बस इतनी देना, कि औरों का भला हो जाये।
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
 LIONEL MY SON
LIONEL MY SON


Posaconazole 泊沙康唑 , بوساكونازول , Позаконазол
Sch-
56592
4-[4-[4-[4-[[(5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)oxolan-3-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2S,3S)-2-hydroxypentan-3-yl]-1,2,4-triazol-3-one
- Noxafil
- SCH 56592
U.S. Patents 5,661,151; 5,703,079; and 6,958,337.
Therap-Cat: Antifungal.
CAS 171228-49-2
Molecular Formula: C37H42F2N8O4
Molecular Weight: 700.78
CAS Name: 2,5-Anhydro-1,3,4-trideoxy-2-C-(2,4-difluorophenyl)-4-[[4-[4-[4-[1-[(1S,2S)-1-ethyl-2-hydroxypropyl]-1,5-dihydro-5-oxo-4H-1,2,4-triazol-4-yl]phenyl]-1-piperazinyl]phenoxy]methyl]-1-(1H-1,2,4-triazol-1-yl)-D-threo-pentitol
Additional Names: (3R-cis)-4-[4-[4-[4-[5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)tetrahydrofuran-3-ylmethoxy]phenyl]piperazin-1-yl]phenyl]-2-[1(S)-ethyl-2(S)-hydroxypropyl]-3,4-dihydro-2H-1,2,4-triazol-3-one
Syn..........Dominic De Souza, "PREPARATION OF POSACONAZOLE INTERMEDIATES." U.S. Patent US20130203994, issued August 08, 2013.
Percent Composition: C 63.41%, H 6.04%, F 5.42%, N 15.99%, O 9.13%
Melting Point
170-172 deg C
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1365Solubility
In water, 0.027 mg/L at 25 deg C (est)
US EPA; Estimation Program Interface (EPI) Suite. Ver.3.12. Nov 30, 2004. Available from, as of Dec 19, 2005:http://www.epa.gov/oppt/exposure/pubs/episuitedl.htm
US5661151 EXP Jul 19, 2019 PRODUCT PATENT
US 5703079 EXP Aug 26, 2014
US8410077 EXPMar 13, 2029
US9023790 EXPJul 4, 2031
US 6958337 EXP Oct 5, 2018
US 8263600 EXPApr 1, 2022
Posaconazole is a triazole antifungal drug[1][2] marketed in the United States, the European Union, and in other countries by Schering-Plough under the trade name Noxafil. In Canada, posaconazole is marketed by Schering-Plough under the trade name Posanol.

Pharmacology
Mode of action
Posaconazole works by disrupting the close packing of acyl chains of phospholipids, impairing the functions of certain membrane-bound enzyme systems such as ATPase and enzymes of the electron transport system, thus inhibiting growth of the fungi. It does this by blocking the synthesis of ergosterol by inhibiting of the enzymelanosterol 14α-demethylase and accumulation of methylated sterol precursors. Posaconazole is significantly more potent at inhibiting 14-alpha demethylase than itraconazole.[3][4][5]
Microbiology
- Candida spp.
- Aspergillus spp.
- Zygomycetes spp.
Pharmacokinetics
Posaconazole is absorbed within three to five hours. It is predominately eliminated through the liver, and has a half life of about 35 hours. Oral administration of posaconazole taken with a high-fat meal exceeds 90% bioavailabilityand increases the concentration by four times compared to fasting state.[6][7]
Clinical use
It is used to treat invasive infections by Candida species,[8] Mucor, and Aspergillus species[9] in severelyimmunocompromised patients.
Clinical evidence for its utility in treatment of invasive disease caused by Fusarium species (fusariosis) is limited.[10]
Two studies suggest posaconazole may be superior to other triazoles, such as fluconazole or itraconazole, in the prevention of invasive fungal infections, although it may cause more serious side effects.[11][12]
There is also some indication that posaconazole may be the most effective treatment for both chronic and acute Chagas disease, showing much better efficacy than benznidazole.[13] Schering-Plough is currently recruiting participants for a phase 2 clinical trial in Argentina to test its efficacy against asymptomatic, chronic Chagas.[14]
SYNTHESIS



................
http://www.google.com/patents/WO2013042138A2?cl=en
HPLC Method of Analysis for Posaconazole:
Posaconazole is analyzed by HPLC using the following conditions: Apparatus: A liquid chromatographic system is to be equipped with variable wavelength UV-detector; Column: Grace Alltima CI 8, 150 x 4.6mm 3μηι or equivalent; Flow rate: 1.0 ml/min; Wavelength: 210 nm; Column Temperature: 28°C; Injection volume: 10
Run time: 60 min; Diluent: Acetonitrile: water (50:50 v/v); Needle wash: Acetonitrile: water (50:50 v/v); Elution: Gradient; Mobile phase-A: Buffer Acetonitrile (90: 10) v/v; Mobile phase- B: Acetonitrile: water (90:10) v/v; Buffer: 1.74 grams of potassium hydrogen phosphate in 1000 ml of water. Adjust pH to 6.5 with diluted orthophosphoric acid and filtered through 0.45μηι Nylon membrane filter paper and sonicate to degas it. PSD method of analysis for Posaconazole:
The particle size distribution of posaconazole compound of formual-1 is measured using the following conditions:
Instrument: Malvern Master sizer 2000; Measuring range: 0.02 to 2000 μπι; Wet sample: Hydro 2000S; Dispersant: Water; Absorption Index: 0; Refractive Index of water: 1.330; Refractive Index of particle: 1.500; Stirrer speed: 2500 rpm; Obscuration range: 10-20%; Sensitivity: Normal; Measurement time: 12 seconds; Background time: 12 seconds; Internal sonication: 3 minutes; (Tip displacement-70%); Measurement repeat: 3 times at zero second interval.
HPLC Method of Analysis for Benzylated Posaconazole:
Benzylated posaconazole is analyzed by HPLC using the following conditions:
Apparatus: A liquid chromatographic system is to be equipped with variable wavelength UV-detector; Column: X-bridge C18, 50X 4.6mm, 3.5um (or) equivalent; Flow rate: 0.8 ml/min; Wavelength: 210 nm; Column Temperature: 40°C; Injection volume: 5 μΐ,; Run time: 35 min; Diluent: Water: Acetonitrile (40:60) v/v; Needle wash: Water: Acetonitrile (40:60) v/v; Elution: Gradient; Mobile phase-A: Buffer Acetonitrile (90: 10) v/v; Mobile phase-B: Acetonitrile: water (90:10) v/v; Buffer: 1.74 grams of potassium hydrogen phosphate dibasic (anhydrous) in 1000 ml of Milli-Q- Water. Adjust its pH to 6.5 with diluted orthophosphoric acid and filtered through 0.22μπι Nylon membrane filter paper and sonicate to degas it. PXRD analysis of crystalline triazole antifungal compound of formula- 1 was carried out using BRUKER/AXS X-Ray diffractometer using Cu Ka radiation of wavelength 1.5406 A° and continuous scan speed of 0.03°/min.
RS/OVI analysis of amorphous posaconazole is carried out on Agilent GC-6850 series-2 with Flame Ionization detector, column AP vac, flow 2 psi and load is 1 μΐ, detector temperature is 260°C and carrier gas is helium.
The process of the present invention is schematically represented as below:
Scheme-I
ormiia- ormua-
Scheme-II:
Formula-16 Scheme-Ill:
HO N N- NH,
Formula-18
Pure POSACONAZOLE
Patent WO2013042138A2
Example-16: Preparation of Posaconazole (Formula- 1)
5N hydrochloric acid (72 ml) and 10% Pd-C (10 g) were added to a solution of 4- (4_(4-(4-(((3Κ,5Κ)-5-(( i H- 1 ,2,4-triazol- 1 -yl)methyl)-5-(2,4-difluorophenyl)tetrahydro furan-3-yl)methoxy)phenyl)piperazin-l-yl)phenyl)-l-((2S,3R)-2-(benzyloxy)pentan-3- yl)-lH-l,2,4-triazol-5(4H)-one compound of formula-21 (42 g) in methanol (420 ml). The reaction mixture was hydrogenated for 5 hours under a hydrogen gas pressure of 4-5 kg/cm2 at 50°. After completion of reaction, the catalyst was filtered off and washed with methanol. pH of the filtrate was adjusted to ~7.0 using 4N sodium hydroxide. Water was added to the reaction mixture and stirred for 2 hours at 25-35°C. Filtered the separated solid and washed with water. The obtained solid was dissolved in acetone (320 ml)and stirred at reflux temperature for 30 minutes. Filtered the undissolved product and added water to the filtrate and stirred the reaction mixture for 4 hours at 25-35°C. Filtered the separated solid and washed with water. Further the solid was recrystallized from isopropyl alcohol to get the title compound. Purity by HPLC: 99.85%; Yield: 75.0%: Chiral purity by HPLC: 99.82%.
......................
Posaconazole, SCH-56592, Noxafil

EP 0736030; JP 1997500658; US 5661151; US 5703079; WO 9517407
Synthesis of intermediate (XX):
The reaction of 2-chloro-2',4'-difluoroacetophenone (I) with sodium
acetate and NaI in DMF gives 2-acetoxy-2',4'-difluoroacetophenone (II),
which by methylenation with methyltriphenylphosphonium bromide and
sodium bis(trimethylsilyl)amide in THF yields
2-(2,4-difluorophenyl)-2-propen-1-ol acetate ester (III). The hydrolysis
of (III) with KOH in dioxane/water affords the corresponding alcohol
(IV), which is regioselectively epoxidized with titanium
tetraisopropoxide and L-(+)-diethyl tartrate in dichloromethane to
(S)-(-)-2-(2,4-difluorophenyl)oxirane-2-methanol (V). The reaction of
(V) with 1,2,4-triazole (VI) in DMF affords
(R)-2-(2,4-difluorophenyl)-3-(1,2,4-triazol-1-yl)propane-1,2-diol (VII),
which is selectively mesylated with methanesulfonyl chloride and
triethylamine to the monomesylate (VIII). The cyclization of (VIII) with
NaH in DMF gives the oxirane (IX), which is condensed with diethyl
malonate (X) by means of NaH in DMSO to yield a mixture of (5R-cis)- and
(5R-trans)-5-(2,4-difluorophenyl)-2-oxo-5-(1,2,4-triazol-1-ylmethyl)
tetrahydrofuran-3-carboxylic acid ethyl ester (XI). The reduction of
(XI) with NaBH4 and LiCl in ethanol affords
(R)-4-(2,4-difluorophenyl)-2-(hydroxymethyl)-5-(1,2,4-triazol-1-yl)
pentane-1,4-diol (XII), which is selectively tosylated with tosyl
chloride and triethylamine in THF to the bistosylate (XIII). The
cyclization of (XIII) by means of NaH in refluxing toluene gives
(5R-cis)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)
tetrahydrofuran-3-methanol tosylate ester (XIV). The reaction of (XIV)
with 1-(4-hydroxyphenyl)-4-(4-nitrophenyl)piperazine (XV) to obtain
compound (XVI), and the following reaction sequence (XVI) to (XVII) to
(XVIII) to (XIX) to
(5R-cis)-4-[4-[4-[4-[5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)tetrahydrofuran-3-ylmethoxy]phenyl]piperazin-1-yl]phenyl-3,4-dihydro-2H-1,2,4-triazol-3-one
(XX) has been performed according to J Med Chem 1984, 27: 894-900.
1. An improved process for the preparation of (3S,5R)-5-(2,4-difluorophi
(iodomethyl)tetrahydrofuran-3-ca boxylic acid compound of formula-7,
Forrnula-7
comprising of the following steps:
Formula-2
with (R)-4-phenyloxazolidin-2-one compound of formula-3
Formula-3
in presence of a suitable activating
agent and a suitable base in a suitable solvent to provide
(R)-3-(4-(2,4-difluorophenyl)pent-4-enoyl)-4-phenyloxazolidin-2-one
compound of formula-4,
Formula-4
b) hydroxymethylating the compound of
formula-4 with 1,3,5-trioxane in presence of a base and a catalyst in a
suitable solvent to provide (R)-3-((S)-4-(2,4-
difluorophenyl)-2-(hydroxymethyl)pent-4-enoyl)-4-phenyloxazolidin-2-one
compound of formula-5,
Formula-5
c) cyclizing the compound of formula-5
in-situ in presence of iodine and a suitable base in a suitable solvent
to provide (R)-3-((3S,5R)-5-(2,4-difluorophenyl)-5-
(iodomethyl)tetrahydrofuran-3 -carbonyl)-4-phenyloxazolidin-2-one
compound of formula-6,
Formula-6
d) hydrolyzing the compound of formula-6
in presence of a suitable aqueous base and hydrogen peroxide in a
suitable solvent to provide (3S,5R)-5-(2,4-
difluorophenyl)-5-(iodomethyl)tetrahydrofuran-3-carboxylic acid compound
of formula-7.
formula-7,
Formula-7
b) reducing the compound of formula-7
with a suitable reducing agent in a suitable solvent to provide
((3R,5R)-5-(2,4-difluorophenyl)-5-(iodomethyl)tetrahydro
furan-3-yl)methanol compound of formula-8,
Formula-8
Example-16: Preparation of Posaconazole (Formula- 1)
5N hydrochloric acid (72 ml) and 10% Pd-C (10 g) were added to a solution of 4- (4_(4-(4-(((3Κ,5Κ)-5-(( i H- 1 ,2,4-triazol- 1 -yl)methyl)-5-(2,4-difluorophenyl)tetrahydro furan-3-yl)methoxy)phenyl)piperazin-l-yl)phenyl)-l-((2S,3R)-2-(benzyloxy)pentan-3- yl)-lH-l,2,4-triazol-5(4H)-one compound of formula-21 (42 g) in methanol (420 ml). The reaction mixture was hydrogenated for 5 hours under a hydrogen gas pressure of 4-5 kg/cm2 at 50°. After completion of reaction, the catalyst was filtered off and washed with methanol. pH of the filtrate was adjusted to ~7.0 using 4N sodium hydroxide. Water was added to the reaction mixture and stirred for 2 hours at 25-35°C. Filtered the separated solid and washed with water. The obtained solid was dissolved in acetone (320 ml)and stirred at reflux temperature for 30 minutes. Filtered the undissolved product and added water to the filtrate and stirred the reaction mixture for 4 hours at 25-35°C. Filtered the separated solid and washed with water. Further the solid was recrystallized from isopropyl alcohol to get the title compound. Purity by HPLC: 99.85%; Yield: 75.0%: Chiral purity by HPLC: 99.82%.
......................
Posaconazole, SCH-56592, Noxafil

EP 0736030; JP 1997500658; US 5661151; US 5703079; WO 9517407

The bromosulfonate (XXI) has been obtained as follows: (S)-Lactic acid methyl ester (XXII) has been protected as its benzyloxymethyl ether (XXIII) according to Tetrahedron Lett 1980, 21: 1035. The reduction of (XXIII) with DIBAL yields the corresponding aldehyde (XXIV), which by a Grignard reaction with ethylmagnesium bromide in THF and chromatographic separation of the diastereoisomers (SiO2, hexane/ethyl acetate) affords 2(S)-(benzyloxymethoxy)-3(R)-pentanol (XXV). Finally, this compound is sulfonated to (XXI) with 4-bromobenzenesulfonyl chloride. Finally, compound (XX) is condensed with 2(S)-(benzyloxymethoxy)-3(R)-pentanol 4-bromobenzenesulfonate ester (XXI) by means of cesium carbonate in DMF, and deprotected with 6N HCl.
.....................
WO 9633178

A new synthesis of Sch-56592 has been described: The reaction of (S)-ethyl lactate (I) with pyrrolidine (II) gives 1-[(S)-lactoyl]pyrrolidine (III), which is benzylated as usual with benzyl chloride yielding the benzyl ether (IV). The reaction of (IV) with ethylmagnesium bromide in THF affords 2(S)-benzyloxy-3-pentanone (V), which is reduced with LiBH4 in dimethoxyethane giving 2(S)-benzyloxy-3(RS)-pentanol (VI). The reaction of (VI) with 4-chlorobenzenesulfonyl chloride (VII) yields the corresponding sulfonate (VIII), which is treated with hydrazine in ethanol to afford a diastereomeric mixture of hydrazines that is resolved with L-dibenzoyltartaric acid giving the (S,S)-enantiomer (IX). The formylation of (IX) with refluxing ethyl formate yields the chiral formyl hydrazide (X), which is cyclized with N-[4-[4-[4-(trimethylsilyloxy)phenyl]piperazin-1-yl]phenyl]carbamic acid phenyl ester (XI) affording the triazolone (XII). Finally, this compound is condensed with the chiral tetrahydrofuran derivative (XIII) by means of NaOH in DMSO, and debenzylated by hydrogenation with H2 over Pd/C in formic acid
.....................
\35th Intersci Conf Antimicrob Agents Chemother (Sept 17-20, San Francisco) 1995,Abst F83.

2) The 2-(2,4-difluorophenyl)-2-propen-1-ol (IV) is converted into the corresponding propenyl bromide (XXVI), which is condensed with diethyl malonate (X) to afford the malonyl derivative (XXVII). The reduction of (XXVII) with NaBH4 and LiCl yields the 1,3-propanediol derivative (XXVIII), which is enantioselectively acetylated with vinyl acetate and Novozyme 435 in acetonitrile yielding the isomeric (S)-monoacetate (XXIX). The cyclization of (XXIX) with iodine and NaHCO3 in acetonitrile affords (5R-cis)-5-(2,4-difluorophenyl)-5-(iodomethyl)tetrahydrofuran-3-methanol acetate ester (XXX), which is condensed with sodium 1,2,4-triazole (XXXI) to give (5R-cis)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl) tetrahydrofuran-3-methanol acetate ester (XXXII). The hydrolysis of (XXXII) with NaOH yields the corresponding methanol (XXXIII), which is finally tosylated to the tosyl ester (XIV), already obtained previously.
.........................
35th Intersci Conf Antimicrob Agents Chemother (Sept 17-20, San Francisco) 1995,Abst F61

3) The Friedel Crafts condensation of m-difluorobenzene (XXXIV) with succinic anhydride (XXXV) gives 4-(2,4-difluorophenyl)-4-oxobutyric acid (XXXVI), which is converted by a Wittig reaction into 4-(2,4-difluorophenyl)-4-pentenoic acid (XXXVII) and subsequently into its acyl chloride (XXXVIII). The condensation of (XXXVIII) with 4(R)-benzyloxazolidin-2-one (XXXIX) gives the acyl oxazolidinone (XL), which is regioselectively hydroxymethylated with 1,3,5-trioxane and TiCl4 to afford 4(R)-benzyl-3-[4-(2,4-difluorophenyl)-3(S)-(hydroxymethyl)-4-pentenoyl] oxazolidin-2-one (XLI). The cyclization of (XLI) with iodine and pyridine yields the tetrahydrofuran derivative (XLII), which is reduced with LiBH4 to (5R-cis)-5-(2,4-difluoromethyl)-5-(iodomethyl)tetrahydrofuran-3-methanol (XLIII). Finally, this compound is condensed with sodium 1,2,4-triazole (XXXI) to afford (5R-cis)-5-(2,4-difluoromethyl)-5-(1,2,4-triazol-1-ylmethyl) tetrahydrofuran-3-methanol (XXXIII), already obtained in Scheme 22656201c.
.................
J Label Compd Radiopharm 1998,41(8),731

The synthesis of [3H]-SCH-51048 has been described: The tritiation of phenol (I) with tritiated heptafluorobutyric acid at 115 C gives the polytritiated intermediate (II), which is then condensed with the chiral tosylate (III) by means of NaOH in DMSO affording labeled SCH-51048.
......................

The synthesis of [14C]-SCH-56592 has been described: The cyclization of semicarbazide (I) with [14C]-formamidine (II) in hot 2-methoxyethanol gives the triazolone (III), which is condensed with the sulfonate (IV) by means of Cs2CO3 in hot DMF to yield the alkylated triazolone (V). Finally, this compound is deprotected by hydrogenation with formic acid over Pd/C in hot methanol to afford labeled SCH-56592.
..................
Tetrahedron Lett 2002,43(18),3359

The condensation of 4-chlorophenylsulfonate (I) with 4-bromophenol (II) by means of K2CO3 in hot DMF gives the aryl ether (III), which is condensed with piperazine (IV) by means of Pdo to yield the monosubstituted piperazine (V). Finally, this compound is condensed with the 4-bromophenyltriazolone (VI) by means of K2CO3 in hot DMSO to afford the target disubstituted piperazine

Alternatively, the condensation of with the 4-bromophenyltriazolone (VI) with piperazine (IV) by means of Pd2(dba)3, BINAP and t-BuONa in hot toluene gives the monosubstituted piperazine (VII), which is then condensed with the already reported aryl ether (III) by means of Pd2(dba)3, BINAP and t-BuONa in hot toluene, and debenzylated with Pd/C and formic acid to afford the target disubstituted piperazine.

The intermediate 4-[4-(4-aminophenyl)piperazin-1-yl]phenol (VIII) has been obtained by several related ways: 1.- The condensation of 4-bromonitrobenzene (I) with piperazine (II) gives 1-(4-nitrophenyl)piperazine (III), which is condensed with 4-bromoanisole (IV) by means of Pdo to yield 1-(4-methoxyphenyl)-4-(4-nitrophenyl)piperazine (V). Alternatively, (V) can also be obtained by condensation of (III) with 4-methoxyphenylboronic acid (VI) by means of Cu(OAc)2 in DMSO. The demethylation of (V) with HBr yields 4-[4-(4-nitrophenyl)piperazin-1-yl]phenol (VII), which is finally reduced with H2 over Pd/C to afford the target 4-[4-(4-aminophenyl)piperazin-1-yl]phenol (VIII) intermediate (see Synthline, scheme no. 22656202a, intermediate (XIV)). 2.- The condensation of piperazine (II) with 4-bromoanisole (IV) by means of Pdo gives 1-(4-methoxyphenyl)piperazine (IX), which is condensed with 4-bromonitrobenzene (I) by means of K2CO3 and tetrabutylammonium iodide (TBAI) in hot DMSO to yield intermediate 1-(4-methoxyphenyl)-4-(4-nitrophenyl)piperazine (V), already reported. 3.- The condensation of piperazine (II) with 4-(benzyloxy)phenyl bromide (X) by means of Pdo gives 1-(4-benzyloxyphenyl)piperazine (XI), which is condensed with 4-bromonitrobenzene (I) by means of K2CO3 and TBAI in hot DMSO to yield 1-(4-benzyloxyphenyl)-4-(4-nitrophenyl)piperazine (XII). The nitro group of (XII) is reduced by means of H2 (50 psi) over Pd/C in wet THF at 50? C to afford 4-[4-(4-benzyloxyphenyl)piperazin-1-yl]aniline (XIII), which is finally debenzylated with H2 (80 psi) over Pd/C in wet THF at 70? C or other drastic conditions to afford the target 4-[4-(4-aminophenyl)piperazin-1-yl]phenol (VIII) intermediate (see Synthline, scheme no. 22656202a, intermediate (XIV)). Alternatively, 1-(4-benzyloxyphenyl)-4-(4-nitrophenyl)piperazine (XII) can also be reduced directly to the target intermediate (VIII) with H2 over Pd/C under a variety of drastic conditions.
Literature References:
Orally active triazole antifungal. Prepn: A. K. Saksena et al., WO 9517407; eidem, US 5661151 (1995, 1997 both to Schering); eidem, Tetrahedron Lett. 37, 5657 (1996).
Comparative antifungal spectrum: A. Cacciapuoti et al., Antimicrob. Agents Chemother. 44, 2017 (2000). Pharmacokinetics, safety and tolerability: R. Courtney et al., ibid. 47, 2788 (2003).
HPLC determn in serum: H. Kim et al., J. Chromatogr. B 738, 93 (2000).
Review of development: A. K. Saksena et al. inAnti-Infectives: Recent Advances in Chemistry and Structure Activity Relationships (Royal Soc. Chem., Cambridge, 1997) pp 180-199; and clinical efficacy in fungal infections: R. Herbrecht, Int. J. Clin. Pract. 58, 612-624 (2004).
References
- ^ Schiller DS, Fung HB (September 2007)."Posaconazole: an extended-spectrum triazole antifungal agent". Clin Ther 29 (9): 1862–86.doi:10.1016/j.clinthera.2007.09.015.PMID 18035188.
- ^ Rachwalski EJ, Wieczorkiewicz JT, Scheetz MH (October 2008). "Posaconazole: an oral triazole with an extended spectrum of activity". Ann Pharmacother 42(10): 1429–38. doi:10.1345/aph.1L005.PMID 18713852.
- ^ a b Brunton L, Lazo J, Parker K. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. San Francisco: McGraw-Hill; 2006. ISBN 978-0-07-142280-2
- ^ "Clinical Pharmacology Pasoconazole". Retrieved18 February 2010.
- ^ "Daily Med, Product Information Noxafil". Retrieved18 February 2010.
- ^ a b Dodds Ashley, Elizabeth; Perfect, John (October 13, 2009). "Pharmacology of azoles". Retrieved18 February 2010.
- ^ "Drugs at FDA: Noxafil" (PDF). Retrieved18 February 2010.
- ^ Li X, Brown N, Chau AS et al. (January 2004)."Changes in susceptibility to posaconazole in clinical isolates of Candida albicans". J. Antimicrob. Chemother. 53 (1): 74–80. doi:10.1093/jac/dkh027.PMID 14657086.
- ^ Walsh TJ, Raad I, Patterson TF et al. (January 2007)."Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial".Clin. Infect. Dis. 44 (1): 2–12. doi:10.1086/508774.PMID 17143808.
- Raad I, Hachem R, Herbrecht R et al. (2006)."Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions". Clin Infect Dis 42(10): 1398–1403.
- Cornely O, Maertens J, Winston D, Perfect J, Ullmann A, Walsh T, Helfgott D, Holowiecki J, Stockelberg D, Goh Y, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D (2007). "Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia". N Engl J Med356 (4): 348–59. doi:10.1056/NEJMoa061094.PMID 17251531.
- Ullmann A, Lipton J, Vesole D, Chandrasekar P, Langston A, Tarantolo S, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S (2007). "Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease". N Engl J Med 356 (4): 335–47. doi:10.1056/NEJMoa061098.PMID 17251530.
- "Am J Trop Med Hyg April 2010 vol. 82 no. 4"
- "A Study of the Use of Oral Posaconazole (POS) in the Treatment of Asymptomatic Chronic Chagas Disease (P05267 AM1) (STOP CHAGAS)"
Noxafil is an azole antifungal agent available as concentrated solution to be diluted before intravenous administration, delayed-release tablet, or suspension for oral administration.
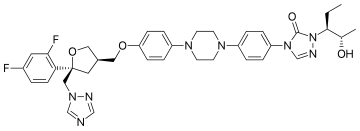
Posaconazole is designated chemically as 4-[4-[4-[4-[[ (3R,5R)-5-(2,4-difluorophenyl)tetrahydro-5(1H-1,2,4-triazol-1-ylmethyl)-3-furanyl]methoxy]phenyl]-1-piperazinyl]phenyl]-2-[(1S,2S)-1-ethyl-2hydroxypropyl]-2,4-dihydro-3H-1,2,4-triazol-3-one with an empirical formula of C37H42F2N8O4 and a molecular weight of 700.8. The chemical structure is:
Posaconazole is a white powder with a low aqueous solubility.

Noxafil injection is available as a clear colorless to yellow, sterile liquid essentially free of foreign matter. Each vial contains 300 mg of posaconazole and the following inactive ingredients: 6.68 g Betadex Sulfobutyl Ether Sodium (SBECD), 0.003 g edetate disodium, hydrochloric acid and sodium hydroxide to adjust the pH to 2.6, and water for injection.
Noxafil delayed-release tablet is a yellow, coated, oblong tablet containing 100 mg of posaconazole. Each delayed-release tablet contains the inactive ingredients: hypromellose acetate succinate, microcrystalline cellulose, hydroxypropylcellulose, silicon dioxide, croscarmellose sodium, magnesium stearate, and Opadry® II Yellow (consists of the following ingredients: polyvinyl alcohol partially hydrolyzed, Macrogol/PEG 3350, titanium dioxide, talc, and iron oxide yellow).
Noxafil oral suspension is a white, cherry-flavored immediate-release suspension containing 40 mg of posaconazole per mL and the following inactive ingredients: polysorbate 80, simethicone, sodium benzoate, sodium citrate dihydrate, citric acid monohydrate, glycerin, xanthan gum, liquid glucose, titanium dioxide, artificial cherry flavor, and purified water.
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
4-(4-(4-(4-(((3R,5R)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)oxolan-3-yl)methoxy)phenyl)piperazin-1-yl)phenyl)-2-((2S,3S)-2-hydroxypentan-3-yl)-1,2,4-triazol-3-one
| |
| Clinical data | |
| Trade names | Noxafil, Posanol |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a607036 |
| Licence data | EMA:Link, US FDA:link |
| Pregnancy category | |
| Legal status |
|
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Bioavailability | High |
| Protein binding | 98 to 99% |
| Metabolism | Hepatic glucuronidation |
| Biological half-life | 16 to 31 hours |
| Excretion | Fecal (77%) and renal (14%) |
| Identifiers | |
| CAS Registry Number | 171228-49-2 |
| ATC code | J02AC04 |
| PubChem | CID: 147912 |
| DrugBank | DB01263 |
| ChemSpider | 130409 |
| UNII | 6TK1G07BHZ |
| KEGG | D02555 |
| ChEBI | CHEBI:64355 |
| ChEMBL | CHEMBL1397 |
| Synonyms | 4-{4-[4-(4-{[(5R)-5-(2,4-difluorophenyl)-5-(1H-1,2,4-triazol-1-ylmethyl)oxolan-3-yl]methoxy}phenyl)piperazin-1-yl]phenyl}-1-[(2S,3S)-2-hydroxypentan-3-yl]-4,5-dihydro-1H-1,2,4-triazol-5-one |
| Chemical data | |
| Formula | C37H42F2N8O4 |
| Molecular mass | 700.778 g/mol |

1H NMR PREDICT
13C NMR PREDICT
COSY PREDICT
| CN101824009A * | May 27, 2010 | Sep 8, 2010 | 北京德众万全药物技术开发有限公司 | Simple preparation method for posaconazole and piperazine intermediate thereof |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| WO2015011224A1 * | Jul 24, 2014 | Jan 29, 2015 | Sandoz Ag | Improved process for the preparation of crystalline form iv of posaconazole |
/////
सुकून उतना ही देना प्रभू, जितने से जिंदगी चल जाये। औकात बस इतनी देना, कि औरों का भला हो जाये।
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
Join me on google plus  Googleplus
Googleplus
 amcrasto@gmail.com
amcrasto@gmail.com
 LIONEL MY SON
LIONEL MY SON
He was only in first standard in school when I was hit by a deadly one in a million spine stroke called acute transverse mylitis, it made me 90% paralysed and bound to a wheel chair, Now I keep him as my source of inspiration and helping millions, thanks to millions of my readers who keep me going and help me to keep my son happy

सुकून उतना ही देना प्रभू, जितने से
जिंदगी चल जाये।
औकात बस इतनी देना,
कि औरों का भला हो जाये।
जिंदगी चल जाये।
औकात बस इतनी देना,
कि औरों का भला हो जाये।




















No comments:
Post a Comment