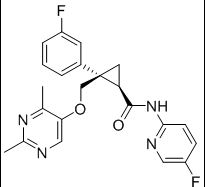
Lemborexant
E2006
CAS Number: 1369764-02-2
MF C22 H20 F2 N4 O2
MW 410.42
Chemical Name: (1R, 2S) -2 – {[(2,4-dimethylpyrimidin-5-yl) oxy] methyl} -2- (3-fluorophenyl ) N (5-fluoropyridin-2-yl) cyclopropanecarboxamide
Cyclopropanecarboxamide, 2-[[(2,4-dimethyl-5-pyrimidinyl)oxy]methyl]-2-(3-fluorophenyl)-N-(5-fluoro-2-pyridinyl)-, (1R,2S)-
(1R,2S)-2-{[(2,4-dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide
Indication: Insomnia
Company: Eisai
Eisai R&D Management Co., Ltd

Lemborexant (INN) (code name E-2006) is a dual antagonist of the orexinOX1 and OX2receptors which is under development byEisai for the treatment of insomnia.[1][2][3] As of December 2014, it is in phase IIclinical trials.[4]
Orexin receptors are G-protein coupled receptors found predominately
in the brain. Their endogenous ligands, orexin-A and orexin-B, are
expressed by neurons localized in the hypothalamus. Orexin-A is a 33
amino acid peptide; orexin-B consists of 28 amino acids. (Sakurai T. et
al., Cell, 1998, 92, 573-585). There are two subtypes of orexin
receptors, OXi and OX2; OX) binds orexin-A preferentially, while OX2
binds both orexin-A and -B. Orexins stimulate food consumption in rats,
and it has been suggested that orexin signaling could play a role in a
central feedback mechanism for regulating feeding behavior (Sakurai et
al., supra). It has also been observed that orexins control wake-sleep
conditions (Chemelli R.M. et al., Cell, 1999, 98, 437-451). Orexins may
also play roles in brain changes associated with opioid and nicotine
dependence (S.L. Borgland et al, Neuron, 2006, 49, 598-601; C.J. Winrow
et al., Neuropharmacology, 2010, 58, 185-194), and ethanol dependence
(J.R. Shoblock et al, Psychopharmacology, 2011, 215, 191-203). Orexins
have additionally been suggested to play a role in some stress reactions
(T. Ida et al, Biochem. Biophys. Res. Commun., 2000, 270, 318- 323).
Compounds such as (lR,2S)-2-(((2,4-dimethylpyrimidin-5-yl)oxy)methyl)-2-(3- fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide (Compound A, below) have been found to be potent orexin receptor antagonists, and may be useful in the treatment of sleep disorders such as insomnia, as well as for other therapeutic uses.
Compounds such as (lR,2S)-2-(((2,4-dimethylpyrimidin-5-yl)oxy)methyl)-2-(3- fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide (Compound A, below) have been found to be potent orexin receptor antagonists, and may be useful in the treatment of sleep disorders such as insomnia, as well as for other therapeutic uses.
……………….
paper
Journal of Medicinal Chemistry (2015), 58(11), 4648-4664.


The orexin/hypocretin receptors are a family of G protein-coupled receptors and consist of orexin-1 (OX1) and orexin-2 (OX2)
receptor subtypes. Orexin receptors are expressed throughout the
central nervous system and are involved in the regulation of the
sleep/wake cycle. Because modulation of these receptors constitutes a
promising target for novel treatments of disorders associated with the
control of sleep and wakefulness, such as insomnia, the development of
orexin receptor antagonists has emerged as an important focus in drug
discovery research. Here, we report the design, synthesis,
characterization, and structure–activity relationships (SARs) of novel
orexin receptor antagonists. Various modifications made to the core
structure of a previously developed compound (–)-5, the
lead molecule, resulted in compounds with improved chemical and
pharmacological profiles. The investigation afforded a potential
therapeutic agent, (1R,2S)-2-{[(2,4-dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide
(E2006), an orally active, potent orexin antagonist. The efficacy was
demonstrated in mice in an in vivo study by using sleep parameter
measurements.
(1R,2S)-2-{[(2,4-dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide
(1R,2S)-2-{[(2,4-Dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide (34)
The title compound was synthesized as a white solid (3.66 g, 56.4% yield) from (1R,2S)-2-{[(2,4-dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)cyclopropanecarboxylic acid 18c by adapting the procedure described for compound 23.
1H NMR (400 MHz, DMSO-d) δ (ppm): 1.46–1.50 (m, 1H), 1.68 (t, J = 6.0 Hz, 1H), 2.01 (s, 3H), 2.36 (s, 3H), 2.59–2.63 (m, 1H), 4.27 (d, J = 10.4 Hz, 1H), 4.66 (d, J = 10.4 Hz, 1H), 7.06–7.11 (m, 1H), 7.37–7.44 (m, 3H), 7.60–7.65 (m, 1H), 7.85–7.89 (m, 1H), 8.11 (s, 1H), 8.30 (d, J = 3.2 Hz, 1H), 11.20 (br s, 1H).
13C NMR (150 MHz, CDCl3)
δ (ppm): 18.7, 18.7, 25.0, 29.0, 34.9, 70.7, 114.5, 114.7, 115.9,
124.2, 125.4, 130.2, 135.5, 138.9, 144.1, 147.3, 149.1, 156.4, 157.0,
159.8, 162.8, 167.9.
HRMS (ESI(+)) calcd for C22H21F2N4O2 [M + H]+, 411.1627; found, 411.1622. Purity: >95%.

………………………….
WO 2013123240
E. Preparation of Compounds of Formula V
((lR,2S)-2-(((2,4-dimethylpyrimidin-5-yI)oxy)methyl)-2-(3-fluorophenyl)-cyclopropyl) methanol (11). ((lR,2S)-2-(3-fluorophenyl)-2-((tosyloxy)methyl)cyclopropyl)metliyl acetate (8, 11.05 g, 0.028 mol, 1.0 equiv.), 2,4-dimethylpyrimidin-5-ol (3.74 g, 0.030 mol, 1.07 equiv.), and cesium carbonate (22.94 g, 1.8 equiv.) were dissolved in ACN (110.5 mL), under nitrogen. The solution was stirred vigorously and heated to 65-70 °C for 2-3 hours. The reaction was monitored by HPLC and TLC (EtO Ac/Heptane = 1/1). Once complete, aqueous 1 N NaOH solution (71.82 mL) was added to the reaction mixture. The reaction mixture was stirred at 20-25 °C for 10-16 h, and was monitored by HPLC and TLC (EtO Ac/Heptane = 1/1). Once the hydrolysis reaction was complete, the reaction mixture was diluted with MTBE (110.50 mL) and stirred for at least 15 min. The aqueous layer was back extracted once with MTBE (55.25 mL). The organic layers were combined and washed once with saturated aqueous NaCl solution (33.15 mL). The solvent was removed under reduced pressure to afford the title compound; ((lR,2S)-2-(((2,4- dimethylpyrimidin-5-yl)oxy)methyl)-2-(3-fluorophenyl)cyclopi pyl)methanol: (11, 8.51 g).
((lR,2S)-2-(((2,4-dimethylpyrimidin-5-yl)oxy)methyl)-2-(3-fluorophenyl)- cyclopropyl)methanol: 1H NMR (500 MHz, DMSO-d6) δ 8.21 (s, 1H), 7.33 (td, J = 8.0, 6.5 Hz, 1H), 7.20 (d, J= 7.9 Hz, 1H), 7.19 – 7.14 (m, 1H), 7.01 (ddd, J= 8.3, 2.6, 1.2 Hz, 1H), 4.63 (t, J = 5.4 Hz, 1H), 4.36 (dd, J= 22.5, 10.5 Hz, 2H), 3.72 – 3.61 (m, 2H), 2.45 (s, 3H), 2.22 (s, 3H), 1.51 – 1.43 (m, 1H), 1.23 (dd, J= 8.9, 5.0 Hz, 1H), 1.01 (dd, J= 6.0, 5.3 Hz, 1H). 13C NMR (126 MHz, DMSO-dfi) δ 162.48 (d, JCF = 243.0 Hz), 158.91, 156.26, 149.51, 147.47 (d, JCF = 7.5 Hz), 139.85, 130.35 (d, JCF = 8.5 Hz), 124.72 (d, JCF = 2.5 Hz), 115.54 (d, JCF = 21.3 Hz), 113.43 (d, JCF = 20.9 Hz), 72.73, 60.70, 29.23, 28.64, 24.94, 18.77, 17.06.
HRMS Calculated for C17H20FN2O2 [M+H]+ 303.1590; found 303.1517.
F. Preparation of Compounds of Formula VII((lR,2S)-2-(((2,4-dimethylpyrimidin-5-yI)oxy)methyl)-2-(3-fluorophenyl)-cyclopropyl) methanol (11). ((lR,2S)-2-(3-fluorophenyl)-2-((tosyloxy)methyl)cyclopropyl)metliyl acetate (8, 11.05 g, 0.028 mol, 1.0 equiv.), 2,4-dimethylpyrimidin-5-ol (3.74 g, 0.030 mol, 1.07 equiv.), and cesium carbonate (22.94 g, 1.8 equiv.) were dissolved in ACN (110.5 mL), under nitrogen. The solution was stirred vigorously and heated to 65-70 °C for 2-3 hours. The reaction was monitored by HPLC and TLC (EtO Ac/Heptane = 1/1). Once complete, aqueous 1 N NaOH solution (71.82 mL) was added to the reaction mixture. The reaction mixture was stirred at 20-25 °C for 10-16 h, and was monitored by HPLC and TLC (EtO Ac/Heptane = 1/1). Once the hydrolysis reaction was complete, the reaction mixture was diluted with MTBE (110.50 mL) and stirred for at least 15 min. The aqueous layer was back extracted once with MTBE (55.25 mL). The organic layers were combined and washed once with saturated aqueous NaCl solution (33.15 mL). The solvent was removed under reduced pressure to afford the title compound; ((lR,2S)-2-(((2,4- dimethylpyrimidin-5-yl)oxy)methyl)-2-(3-fluorophenyl)cyclopi pyl)methanol: (11, 8.51 g).
((lR,2S)-2-(((2,4-dimethylpyrimidin-5-yl)oxy)methyl)-2-(3-fluorophenyl)- cyclopropyl)methanol: 1H NMR (500 MHz, DMSO-d6) δ 8.21 (s, 1H), 7.33 (td, J = 8.0, 6.5 Hz, 1H), 7.20 (d, J= 7.9 Hz, 1H), 7.19 – 7.14 (m, 1H), 7.01 (ddd, J= 8.3, 2.6, 1.2 Hz, 1H), 4.63 (t, J = 5.4 Hz, 1H), 4.36 (dd, J= 22.5, 10.5 Hz, 2H), 3.72 – 3.61 (m, 2H), 2.45 (s, 3H), 2.22 (s, 3H), 1.51 – 1.43 (m, 1H), 1.23 (dd, J= 8.9, 5.0 Hz, 1H), 1.01 (dd, J= 6.0, 5.3 Hz, 1H). 13C NMR (126 MHz, DMSO-dfi) δ 162.48 (d, JCF = 243.0 Hz), 158.91, 156.26, 149.51, 147.47 (d, JCF = 7.5 Hz), 139.85, 130.35 (d, JCF = 8.5 Hz), 124.72 (d, JCF = 2.5 Hz), 115.54 (d, JCF = 21.3 Hz), 113.43 (d, JCF = 20.9 Hz), 72.73, 60.70, 29.23, 28.64, 24.94, 18.77, 17.06.
HRMS Calculated for C17H20FN2O2 [M+H]+ 303.1590; found 303.1517.
(lR,2S)-2-(((2,4-dimethylpyrimidin-5-yl)oxy)methyl)-2-(3-fluorophenyl)cyclopropane- carboxylic acid (13). ((lR,2S)-2-(((2,4-dimethylpyrimidin-5-yl)oxy)methyl)-2-(3- fluorophenyl)cyclopropyl)methanol (11, 87.5 g, 290 mmol, 1.0 equiv.) was dissolved in toluene (390 mL). To the mixture was added pH 7 buffer (107 g, prepared from 4.46 g of sodium phosphate dibasic and 7.79 g of sodium phosphate monobasic in 94.4 mL of water) and 2,2,6,6- tetramethylpiperidine 1-oxyl (TEMPO) (0.93 g, 5.9 mmol, 0.02 equiv.). The mixture was cooled to 0 °C and sodium hypochlorite solution (5% active chlorine, 383 mL, 304 mmol, 1.05 equiv.) was added dropwise, maintaining the internal temperature below 9 °C. The mixture was allowed to warm to room temperature and stirred for 2 h. To the mixture was added aqueous hydrochloric acid (2.0 M, 8.73 mL, 0.05 equiv.) followed by a solution of sodium chlorite (36.0 g, 318 mmol, 1.1 equiv.) in water (87 mL), maintaining the internal temperature below 26 °C. The mixture was stirred at room temperature for 4 h, and then cooled to 10 °C. A solution of sodium thiosulfate (92 g, 579 mmol, 2.0 equiv.) in water (177 mL) was added, maintaining the internal temperature below 20 °C. The mixture was stirred for 20 min, and then aqueous sodium hydroxide solution (4 N, 87 mL, 348 mmol, 1.2 equiv.) was added to achieve ca. pH = 13. The mixture was heated to 80 °C for 4 hours, then cooled to room temperature. Stirring was halted and the phases allowed to split. The lower aqueous phase was collected and the upper organic phase was washed once with 4 N sodium hydroxide solution (17 mL). The combined aqueous phases were acidified with aqueous hydrochloric acid solution (4 N, 17 mL) to pH = 4 and extracted with ethyl acetate (2 x 470 mL). The combined organic phases were washed with ca. 20% aqueous NaCl solution (175 mL). The organic phases were concentrated by rotary evaporation to yield 96.84 g of crude oil. A portion (74 g) of this crude oil was dissolved in acetonitrile (400 mL) and concentrated to dryness by rotary evaporation. Another portion of acetonitrile (400 mL) was added and the mixture was again concentrated to dryness. To the residue was added acetonitrile (370 mL). The mixture was heated to 65 °C resulting in a clear solution. The mixture was cooled to room temperature, then to 0 °C and held at this temperature for 6 h. The mixture was filtered and the wet cake was washed with acetonitrile (2 x 74 mL). The cake was dried under vacuum with a nitrogen sweep, then in a vacuum oven at 20 torr and 40 °C to afford (lR,2S)-2-(((2,4- dimethylpyrimidin-5-yl)oxy)methyl)-2-(3-fluorophenyl)cyclopropanecarboxylic acid (13, 56.9 g, 80% yield) as an off-white crystalline solid.
(lR,2S)-2-(((2,4-dimethylpyrimidin-5-yl)oxy)methyl)-2-(3-fluoi phenyl)- cyclopropanecarboxylic acid: 1H NMR (500 MHz, DMSO-d6) δ 12.47 (s, 1H), 8.17 (s, 1H), 7.39 (td, J= 8.0, 6.4 Hz, 1H), 7.29 (d, J= 7.9 Hz, 1H), 7.27 – 7.22 (m, 1H), 7.10 (td, J – 8.3, 2.1 Hz, 1H), 4.63 (d, J= 10.2 Hz, 1H), 4.30 (d, J= 10.2 Hz, 1H), 2.46 (s, 3H), 2.26 (s, 3H), 2.13 (dd, J= 7.7, 6.6 Hz, 1H), 1.63 – 1.54 (m, 2H); 13C NMR (126 MHz, DMSO-d6) δ 172.65, 162.48 (d, JCF = 243.6 Hz), 159.08, 156.24, 149.45, 145.15 (d, JCF = 7.5 Hz), 139.60, 130.71 (d, JCF = 8.5 Hz), 124.79 (d, JCF = 2.6 Hz), 115.60 (d, JCF = 21.8 Hz), 114.32 (d, JCF = 20.8 Hz), 71.15, 33.92 (d, JCF = 2.0 Hz), 26.46, 24.96, 19.72, 18.70.
HRMS Calculated for Ci7Hi8FN203 [M+H]+ 317.1301; found 317.1298.
G. Preparation of Compounds of Formula IX
(lR,2S)-2-(((2,4-dimethylpyrimidin-5-yl)oxy)methyl)-2-(3-fluorophenyl)-N-(S- fluoropyridin-2-yl)cyclopropanecarboxamide (14). (lR,2S)-2-(((2,4-dimethylpyrimidin- 5-yl)oxy)methyl)-2-(3-fluorophenyl)-cyclopropanecarboxylic acid (13, 12.80 g, 0.040 mol, 1.0 equiv.), and 2-amino-5-fluoiOpyridine (4.76 g, 0.0425 mol, 1.05 equiv.) were dissolved in ethyl acetate (102.4 mL), under nitrogen. The solution was cooled to 0-5 °C, and N,N- diisopropylethylamine (14.10 mL, 0.081 mol, 2.0 equiv.) was added to the reaction mixture while maintaining the internal temperature at 0-15 °C. The reaction mixture was stirred at 0-10 °C for 20-30 minutes. n-Propylphosphonic anhydride (T3P; 50% w/w solution in ethyl acetate, 36.1 g, 1.4 equiv.) was added to the reaction mixture while maintaining the internal temperature at 0-15 °C. The reaction was stirred at 20-25 °C for at least 20-24 hour and monitored by HPLC and TLC (EtO Ac/Heptane = 1/1). Upon completion of the reaction, the reaction mixture was cooled to 0-5 °C and then was quenched with water (64.0 mL) while maintaining the internal temperature below 10-15 °C. The aqueous layer was back extracted once with MTBE (76.8 mL). The organic layers were combined and washed once with saturated aqueous NaHC03 solution (38.4 mL) and once with water (38.4 mL). The organic layer was polish filtered and the filter rinsed with MTBE (12,8 mL). The organic layer was then concentrated under reduced pressure to a minimum stirrable volume. Ethyl acetate (60.8 mL) was added to the reaction mixture and the mixture was heated to no more than 50 °C to achieve a clear solution. n-Heptane (86.3 mL) was added slowly with agitation. The reaction mixture was cooled to 20-25 °C, and the suspension was stirred for at least 1 h at 20-25 °C and then stirred at least for 1 h at 0-5 °C. The suspension was filtered and the cake was washed two times with 5 : 1 heptane/ethyl acetate (2 x
12.8 mL). The cake was dried under nitrogen and/or vacuum to provide the title compound, (lR,2S)-2-(((2,4-dimethylpyrimidin-5-yl)oxy)methyl)-2-(3-fluoiOphenyl)-N-(5-fiuoropyridin^ yl)cyclopropanecarboxamide, (14, 12.54 g, >99% ee) as a white to off white solid.
(lR,2S)-2-(((2,4-dimethylpyrimidin-5-yl)oxy)methyl)-2-(3-fluoiOphenyl)-N-(5- fluoropyridin-2-yl)cyclopropanecarboxamide:
1H NMR (500 MHz, DMSO-d6) δ 11.19 (s, 1H), 8.31 (d, J = 3.0 Hz, 1H), 8.12 (s, 1H), 7.94 – 7.85 (m, 1H), 7.62 (tt, J = 8.7, 3.1 Hz, 1H), 7.44 (dd, J = 10.6, 1.5 Hz, 1H), 7.41 – 7.40 (m, 1H), 7.39 (s, 1H), 7.14 – 7.06 (m, 1H), 4.67 (d, J = 10.2 Hz, 1H), 4.29 (t, J= 9.9 Hz, 1H), 2.63 (t, J= 7.0 Hz, 1H), 2.38 (s, 3H), 2.03 (s, 3H), 1.76 – 1.64 (m, 1H), 1.49 (dd, J = 8.0, 4.8 Hz, 1H); 13C NMR (125 MHz, DMSO-d6) δ 168.68, 161.98 (d, JcF = 242.3 Hz), 158.46, 155.15, 155.38 (d, JCF = 247.9 Hz), 148.90, 148.51, 145.00 (d, JCF = 7.7 Hz), 139.37, 135.15 (d, JCF = 24.9 Hz), 130.06 (d, JCF = 8.4 Hz), 125.05 (d, JCF = 19.5 Hz), 124.70 (d, JCF = 2.6 Hz), 115.71 (d, JCF = 21.7 Hz), 114.20 (d, JCF = 4.1 Hz), 113.70 (d, JCF =
20.9 Hz), 70.80, 34.09 (d, JCF = 1.9 Hz), 26.90, 24.38, 18.37, 17.78.
HRMS Calculated for C22H21F2N402 [M+H]+ 411.1627; found 411.1632.
……………….
WO 2012039371
Production Example 14
(1R, 2S) -2 – Synthesis of {[(2,4-dimethyl-pyrimidin-5-yl) oxy] methyl} -2- (3-fluorophenyl) cyclopropanecarboxylic acid (Prep14-6)
(1) (1S, 5R) -1- (3- fluorophenyl) -3-hexane-2-one to oxabicyclo [3.1.0] (Prep14-1)
3-fluorophenyl acetonitrile (70g) was dissolved in THF (500ml), ice – salt bath under cooling, was added dropwise NaHMDS (1000ml, 1.06M). After allowed to stir 1 hour, R – (-) – it was added dropwise epichlorohydrin (40.6ml) (approximately 10 minutes, the internal temperature <10 ℃). After it was allowed to stirred for 2 hours (maintained before and after the internal temperature 0 ℃), and stirred at room temperature for 14 hours. The reaction was I was dropping a small amount of water cooled with ice. The reaction solution was concentrated under reduced pressure, the residue in ethanol (700ml), 1N potassium hydroxide aqueous solution (1000ml) was added and heated to reflux for 5 hours. After returning to room temperature, it was added 5N hydrochloric acid (400ml), and stirred for 1 hour at 60 ℃. The reaction mixture was concentrated under reduced pressure, it was added thereto to carry out a liquid separation with ethyl acetate and water. The organic layer saturated aqueous sodium hydrogen carbonate solution, it was washed successively with saturated sodium chloride aqueous solution. Dried over magnesium sulfate, and the solvent was concentrated under reduced pressure. The residue was purified by silica gel column chromatography to obtain a purified by (n- heptane-ethyl acetate) The title compound (84.9g).
1 H-NMR (400MHz, CDCl 3) δ (ppm): 1.41 (t, J = 5.2Hz, 1H), 1.64 (dd, J = 8.0,5.2Hz, 1H), 2 .56-2.63 (m, 1H), 4.30 (d, J = 9.2Hz, 1H), 4.47 (dd, J = 9.2,4.8Hz, 1H), 6.96- 7.02 (m, 1H), 7.16-7.21 (m, 2H), 7.28-7.35 (m, 1H).
(2) (1S, 2R) -1- (3- fluorophenyl) cyclopropane-1,2-dimethanol (Prep14-2)
THF- methanol compound Prep14-1 (72.7g) (440ml-220ml) sodium borohydride solution (25g) was added at 0 ℃, and the mixture was stirred for 65 hours at room temperature. Under ice-cooling, water and 5N hydrochloric acid were added to the reaction solution, followed by extraction with ethyl acetate. The organic layer was washed with a saturated sodium chloride aqueous solution, and then dried with magnesium sulfate. The solvent was concentrated under reduced pressure, the residue was purified by silica gel column chromatography to obtain a purified by (n- heptane-ethyl acetate) The title compound (72.7g).
1 H-NMR (400MHz, CDCl 3) δ (ppm): 0.80 (t, J = 5.0Hz, 1H), 1.10 (dd, J = 8.6,5.0Hz, 1H), 1 .62-1.71 (m, 1H), 3.41 (t, J = 11.4Hz, 1H), 3.58 (d, J = 12.0Hz, 1H), 4.12-4.25 ( m, 2H), 6.90-6.96 (m, 1H), 7.08-7.14 (m, 1H), 7.16-7.21 (m, 1H) 7.24-7.32 (m, 1H).
(3) {(1S, 2R) – [2- (tert- butyldiphenylsilyloxy) -1- (3-fluorophenyl) cyclopropyl]} methanol (Prep14-3)
Compound Prep14-2 a (42.4g) was dissolved triethylamine (33.0ml) in dichloromethane (216ml), was cooled to -20 ℃, was added dropwise tert- butyldiphenylsilyl chloride (56.3ml) (about 30 minute, almost at the same time insoluble matter is deposited with the completion of the dropping). After stirring for 1 hour, further stirred at room temperature for 20 hours.Water was added to the reaction mixture, and the mixture was extracted with dichloromethane. Washed with water and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography to obtain a purified by (n- heptane ethyl acetate) The title compound (67.8g).
1 H-NMR (400MHz, CDCl 3) δ (ppm): 0.73 (t, J = 5.2Hz, 1H), 1.04 (dd, J = 8.4,5.2Hz, 1H), 1 .09 (s, 9H), 1.48-1.53 (m, 1H), 3.52 (t, J = 12.0Hz, 1H), 3.56 (dd, J = 9.6,1. 6Hz, 1H), 3.70 (dd, J = 9.6,1.6Hz, 1H), 4.18 (t, J = 12.0Hz, 1H), 4.20 (dd, J = 12.0 , 5.2Hz, 1H), 6.93 (tdd, J = 8.0,2.4,1.2Hz, 1H), 7.11 (dt, J = 9.6,2.4Hz, 1H), 7.20 (dt, J = 8.0,1.2Hz, 1H), 7.28 (td, J = 8.0,6.0Hz, 1H), 7.37-7.49 (m, 6H) , 7.69-7.74 (m, 4H).
(4) {(1R, 2S) -2 – {[(-5- 2,4- dimethyl-pyrimidin-yl) oxy] methyl} -2- (3-fluorophenyl) cyclopropyl} methanol (Prep14-4)
Compound Prep14-3 (581mg), triphenylphosphine (1.3g) and Preparation Example 4 to give 2,4-dimethyl – THF (10ml) solution of diisopropyl azodicarboxylate pyrimidin-5-ol (183mg) ( The 0.316ml) was added dropwise at 0 ℃, and the mixture was stirred at room temperature for 2 days. The reaction mixture was concentrated under reduced pressure, silica gel column chromatography (n- heptane: ethyl acetate = 19: 1 → 7: 3) was purified by. The resulting (1S, 2R) -2- (tert- butyldiphenylsilyloxy-methyl) -1 – {[(2,4-dimethyl-pyrimidin-5-yl) oxy] methyl} -1- (3-fluorophenyl) cyclopropane was dissolved in THF (15ml), tetrabutylammonium fluoride (1M-THF solution: 1.61ml) was added dropwise at room temperature and stirred at room temperature for 14 hours. The reaction mixture was concentrated under reduced pressure, silica gel column chromatography (n- heptane: ethyl acetate = 10: 1 → 0: 1) to obtain purified by the title compound (238mg).
1 H-NMR (400MHz, CDCl 3) δ (ppm): 1.00 (t, J = 5.6Hz, 1H), 1.25-1.33 (m, 1H), 1.78-1.88 (m, 1H), 2.39 (s, 3H), 2.61 (s, 3H), 3.58 (dd, J = 12.0,9.6Hz, 1H), 4.02-4.11 (m, 1H), 4.12 (d, J = 10.4Hz, 1H), 4.43 (d, J = 9.6Hz, 1H), 6.92-6.98 (m, 1H), 7 .10-7.16 (m, 1H), 7.18-7.23 (m, 1H), 7.29 (td, J = 8.0,6.0Hz, 1H), 8.00 (s, 1H).
(4 alternative method)
((1R, 2S) -2 – {[(2,4- dimethyl-pyrimidin-5-yl) oxy]} methyl] -2- (3-fluorophenyl) cyclopropyl} methanol (Prep14-4) (alternative method)
Triethylamine (14.5ml) was added in dichloromethane (200ml) solution of compound Prep14-3 (41.3g), cooled to 0 ℃. It was added dropwise methanesulfonyl chloride (7.34ml), and stirred for 1 hour. Water was added to the reaction mixture, and the mixture was extracted with dichloromethane. Dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The resulting residue in acetonitrile (200ml) solution obtained in Production Example 4- (2) 2,4-dimethyl – pyrimidin-5-ol (14.1g) and cesium carbonate (61.8g) was added, 70 ℃ It was heated to. After 4 hours of stirring at 70 ℃, the reaction solution was cooled to 0 ℃, tetrabutylammonium fluoride (1M-THF solution: 190ml) was added dropwise, and the mixture was stirred for 1 hour at room temperature. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. Dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by NH- silica gel column chromatography (n- heptane: ethyl acetate = 9: 1 to 1: 1) to give the title compound (20.7g) was purified by.
(5) (1R, 2S) -2 – {[(2,4- dimethyl-pyrimidin-5-yl) oxy] methyl} -2- of (3-fluorophenyl) cyclopropane carbaldehyde (Prep14-5)
Oxalyl dichloromethane solution of chloride (137μl) a (7ml) was cooled to -78 ℃, there was added dropwise dimethyl sulfoxide (226μl) (internal temperature below -60 ℃). After stirring for 10 minutes at the same temperature, dichloromethane (3ml) solution of the compound to the reaction mixture Prep14-4 (238mg) was dropped at -78 ℃, and the mixture was stirred at the same temperature for 30 minutes. After stirring for 15 minutes triethylamine (671μl) was added to the reaction mixture, and the temperature was raised to room temperature. Saturated sodium chloride aqueous solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was dried anhydrous magnesium sulfate and concentrated under reduced pressure to give the crude title compound (236mg).
1 H-NMR (400MHz, CDCl 3) δ (ppm): 1.67 (dd, J = 8.0,4.8Hz, 1H), 1.96-2.00 (m, 1H), 2.36 (s, 3H), 2.49-2.55 (m, 1H), 2.59 (s, 3H), 4.19 (d, J = 9.6Hz, 1H), 4.44 (d, J = 10.0Hz, 1H), 6.97-7.04 (m, 1H), 7.14-7.20 (m, 1H), 7.21-7.25 (m, 1H), 7.30 -7.37 (m, 1H), 7.95 (s, 1H), 9.87 (d, J = 3.2Hz, 1H).
(6) (1R, 2S) -2 – {[(2,4- dimethyl-pyrimidin-5-yl) oxy] methyl} -2- (3-fluorophenyl) cyclopropanecarboxylic acid (Prep14-6)Compound Prep14- 5 (18.9g) and 2-methyl-2-butene (26.1ml), sodium dihydrogen phosphate the (9.07g) was dissolved in acetone-water mixed solvent (200ml · 40ml), sodium chlorite ( 6.26g) and I were added little by little. After stirring for 2 hours at room temperature, the reaction solution was concentrated under reduced pressure. The precipitated solid was filtered off, washed with dichloromethane, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel column chromatography (n- heptane: After 1, ethyl acetate: ethyl acetate = 1: 1-0 methanol = 10: 1) to give the title compound (16.2g) was purified by.
1 H-NMR (400MHz, CDCl 3) δ (ppm): 1.55 (dd, J = 8.4,5.6Hz, 1H), 1.76 (t, J = 5.6Hz, 1H), 2 .25 (dd, J = 8.4,6.4Hz, 1H), 2.33 (s, 3H), 2.55 (s, 3H), 4.47 (t, J = 9.6Hz, 1H) , 4.50 (d, J = 9.6Hz, 1H), 6.99 (tdd, J = 8.0,2.4,1.2Hz, 1H), 7.21 (dt, J = 9.6 , 2.4Hz, 1H), 7.26 (td, J = 8.0,1.2Hz, 1H), 7.32 (td, J = 8.0,6.0Hz, 1H), 8.21 ( s, 1H).
Compound Prep14-6 can be prepared directly by the following method from the compound Prep14-4.
Compound Prep14-4 (300mg) and TEMPO (5mol%, 7.74mg) was dissolved in phosphate buffer solution of acetonitrile · pH6.4 (5ml · 5ml), 2N- hydrochloric acid (150μl), sodium chlorite (180mg ) and it was added. After heating to 40 °, 5w% of the hypochlorite solution (2mol%, 26.5μl) were added and stirred for 2 hours. Cooled to room temperature, the reaction mixture was stirred for 5 minutes was added an excess of 2-methyl-2-butene in. The reaction solution was extracted with dichloromethane, the solvent was distilled off under reduced pressure, the residue was purified by silica gel column chromatography (n- heptane: ethyl acetate = 1: 1 to 0: After 1, ethyl acetate: methanol = 9: 1) in was purified to give the title compound (215mg).
Example 95
(1R, 2S) -2 – {[(2,4- dimethyl-pyrimidin-5-yl) oxy] methyl} -2- (3-fluorophenyl) -N- (5- fluoro-2-yl) cyclopropane The synthesis of carboxamide (95)
Acid Prep14-6 a (226mg) was dissolved in dichloromethane (10ml), oxalyl chloride (122μl), and stirred for 1 hour at room temperature was added DMF (a few drops). The reaction mixture was concentrated under reduced pressure to give the crude acid chloride. N in THF (10ml) solution of 2-amino-5-fluoro pyridine (96.1mg), N- diisopropylethylamine (283μl) was added mixture was heated to 60 ℃, the temperature of intact dropwise a THF solution of the crude acid chloride in it was allowed to stir for 1 hour. The reaction mixture was allowed to cool to room temperature and allowed to stir for 1 hour, after which the reaction mixture was concentrated under reduced pressure, partitioned between ethyl acetate and water, the organic layer was separated. The organic layer was dried over anhydrous magnesium sulfate, and the filtrate was concentrated under reduced pressure. The residue was purified by NH- silica gel column chromatography (n- heptane: ethyl acetate = 2: 1) to give diethyl ether to the obtained target compound was added. The precipitated solid was filtered dried to give the title compound (130mg).
1 H-NMR (400MHz, d-DMSO) δ (ppm): 1.46-1.50 (m, 1H), 1.68 (t, J = 6.0Hz, 1H), 2.01 (s, 3H), 2.36 (s, 3H), 2.59-2.63 (m, 1H), 4.27 (d, J = 10.4Hz, 1H), 4.66 (d, J = 10. 4Hz, 1H), 7.06-7.11 (m, 1H), 7.37-7.44 (m, 3H), 7.60-7.65 (m, 1H), 7.85-7. 89 (m, 1H), 8.11 (s, 1H), 8.30 (d, J = 3.2Hz, 1H), 11.20 (brs, 1H)
MS [M + H] + = 411
(1R, 2S) -2 – Synthesis of {[(2,4-dimethyl-pyrimidin-5-yl) oxy] methyl} -2- (3-fluorophenyl) cyclopropanecarboxylic acid (Prep14-6)
(1) (1S, 5R) -1- (3- fluorophenyl) -3-hexane-2-one to oxabicyclo [3.1.0] (Prep14-1)
3-fluorophenyl acetonitrile (70g) was dissolved in THF (500ml), ice – salt bath under cooling, was added dropwise NaHMDS (1000ml, 1.06M). After allowed to stir 1 hour, R – (-) – it was added dropwise epichlorohydrin (40.6ml) (approximately 10 minutes, the internal temperature <10 ℃). After it was allowed to stirred for 2 hours (maintained before and after the internal temperature 0 ℃), and stirred at room temperature for 14 hours. The reaction was I was dropping a small amount of water cooled with ice. The reaction solution was concentrated under reduced pressure, the residue in ethanol (700ml), 1N potassium hydroxide aqueous solution (1000ml) was added and heated to reflux for 5 hours. After returning to room temperature, it was added 5N hydrochloric acid (400ml), and stirred for 1 hour at 60 ℃. The reaction mixture was concentrated under reduced pressure, it was added thereto to carry out a liquid separation with ethyl acetate and water. The organic layer saturated aqueous sodium hydrogen carbonate solution, it was washed successively with saturated sodium chloride aqueous solution. Dried over magnesium sulfate, and the solvent was concentrated under reduced pressure. The residue was purified by silica gel column chromatography to obtain a purified by (n- heptane-ethyl acetate) The title compound (84.9g).
1 H-NMR (400MHz, CDCl 3) δ (ppm): 1.41 (t, J = 5.2Hz, 1H), 1.64 (dd, J = 8.0,5.2Hz, 1H), 2 .56-2.63 (m, 1H), 4.30 (d, J = 9.2Hz, 1H), 4.47 (dd, J = 9.2,4.8Hz, 1H), 6.96- 7.02 (m, 1H), 7.16-7.21 (m, 2H), 7.28-7.35 (m, 1H).
(2) (1S, 2R) -1- (3- fluorophenyl) cyclopropane-1,2-dimethanol (Prep14-2)
THF- methanol compound Prep14-1 (72.7g) (440ml-220ml) sodium borohydride solution (25g) was added at 0 ℃, and the mixture was stirred for 65 hours at room temperature. Under ice-cooling, water and 5N hydrochloric acid were added to the reaction solution, followed by extraction with ethyl acetate. The organic layer was washed with a saturated sodium chloride aqueous solution, and then dried with magnesium sulfate. The solvent was concentrated under reduced pressure, the residue was purified by silica gel column chromatography to obtain a purified by (n- heptane-ethyl acetate) The title compound (72.7g).
1 H-NMR (400MHz, CDCl 3) δ (ppm): 0.80 (t, J = 5.0Hz, 1H), 1.10 (dd, J = 8.6,5.0Hz, 1H), 1 .62-1.71 (m, 1H), 3.41 (t, J = 11.4Hz, 1H), 3.58 (d, J = 12.0Hz, 1H), 4.12-4.25 ( m, 2H), 6.90-6.96 (m, 1H), 7.08-7.14 (m, 1H), 7.16-7.21 (m, 1H) 7.24-7.32 (m, 1H).
(3) {(1S, 2R) – [2- (tert- butyldiphenylsilyloxy) -1- (3-fluorophenyl) cyclopropyl]} methanol (Prep14-3)
Compound Prep14-2 a (42.4g) was dissolved triethylamine (33.0ml) in dichloromethane (216ml), was cooled to -20 ℃, was added dropwise tert- butyldiphenylsilyl chloride (56.3ml) (about 30 minute, almost at the same time insoluble matter is deposited with the completion of the dropping). After stirring for 1 hour, further stirred at room temperature for 20 hours.Water was added to the reaction mixture, and the mixture was extracted with dichloromethane. Washed with water and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure, and the residue was purified by silica gel column chromatography to obtain a purified by (n- heptane ethyl acetate) The title compound (67.8g).
1 H-NMR (400MHz, CDCl 3) δ (ppm): 0.73 (t, J = 5.2Hz, 1H), 1.04 (dd, J = 8.4,5.2Hz, 1H), 1 .09 (s, 9H), 1.48-1.53 (m, 1H), 3.52 (t, J = 12.0Hz, 1H), 3.56 (dd, J = 9.6,1. 6Hz, 1H), 3.70 (dd, J = 9.6,1.6Hz, 1H), 4.18 (t, J = 12.0Hz, 1H), 4.20 (dd, J = 12.0 , 5.2Hz, 1H), 6.93 (tdd, J = 8.0,2.4,1.2Hz, 1H), 7.11 (dt, J = 9.6,2.4Hz, 1H), 7.20 (dt, J = 8.0,1.2Hz, 1H), 7.28 (td, J = 8.0,6.0Hz, 1H), 7.37-7.49 (m, 6H) , 7.69-7.74 (m, 4H).
(4) {(1R, 2S) -2 – {[(-5- 2,4- dimethyl-pyrimidin-yl) oxy] methyl} -2- (3-fluorophenyl) cyclopropyl} methanol (Prep14-4)
Compound Prep14-3 (581mg), triphenylphosphine (1.3g) and Preparation Example 4 to give 2,4-dimethyl – THF (10ml) solution of diisopropyl azodicarboxylate pyrimidin-5-ol (183mg) ( The 0.316ml) was added dropwise at 0 ℃, and the mixture was stirred at room temperature for 2 days. The reaction mixture was concentrated under reduced pressure, silica gel column chromatography (n- heptane: ethyl acetate = 19: 1 → 7: 3) was purified by. The resulting (1S, 2R) -2- (tert- butyldiphenylsilyloxy-methyl) -1 – {[(2,4-dimethyl-pyrimidin-5-yl) oxy] methyl} -1- (3-fluorophenyl) cyclopropane was dissolved in THF (15ml), tetrabutylammonium fluoride (1M-THF solution: 1.61ml) was added dropwise at room temperature and stirred at room temperature for 14 hours. The reaction mixture was concentrated under reduced pressure, silica gel column chromatography (n- heptane: ethyl acetate = 10: 1 → 0: 1) to obtain purified by the title compound (238mg).
1 H-NMR (400MHz, CDCl 3) δ (ppm): 1.00 (t, J = 5.6Hz, 1H), 1.25-1.33 (m, 1H), 1.78-1.88 (m, 1H), 2.39 (s, 3H), 2.61 (s, 3H), 3.58 (dd, J = 12.0,9.6Hz, 1H), 4.02-4.11 (m, 1H), 4.12 (d, J = 10.4Hz, 1H), 4.43 (d, J = 9.6Hz, 1H), 6.92-6.98 (m, 1H), 7 .10-7.16 (m, 1H), 7.18-7.23 (m, 1H), 7.29 (td, J = 8.0,6.0Hz, 1H), 8.00 (s, 1H).
(4 alternative method)
((1R, 2S) -2 – {[(2,4- dimethyl-pyrimidin-5-yl) oxy]} methyl] -2- (3-fluorophenyl) cyclopropyl} methanol (Prep14-4) (alternative method)
Triethylamine (14.5ml) was added in dichloromethane (200ml) solution of compound Prep14-3 (41.3g), cooled to 0 ℃. It was added dropwise methanesulfonyl chloride (7.34ml), and stirred for 1 hour. Water was added to the reaction mixture, and the mixture was extracted with dichloromethane. Dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The resulting residue in acetonitrile (200ml) solution obtained in Production Example 4- (2) 2,4-dimethyl – pyrimidin-5-ol (14.1g) and cesium carbonate (61.8g) was added, 70 ℃ It was heated to. After 4 hours of stirring at 70 ℃, the reaction solution was cooled to 0 ℃, tetrabutylammonium fluoride (1M-THF solution: 190ml) was added dropwise, and the mixture was stirred for 1 hour at room temperature. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. Dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by NH- silica gel column chromatography (n- heptane: ethyl acetate = 9: 1 to 1: 1) to give the title compound (20.7g) was purified by.
(5) (1R, 2S) -2 – {[(2,4- dimethyl-pyrimidin-5-yl) oxy] methyl} -2- of (3-fluorophenyl) cyclopropane carbaldehyde (Prep14-5)
Oxalyl dichloromethane solution of chloride (137μl) a (7ml) was cooled to -78 ℃, there was added dropwise dimethyl sulfoxide (226μl) (internal temperature below -60 ℃). After stirring for 10 minutes at the same temperature, dichloromethane (3ml) solution of the compound to the reaction mixture Prep14-4 (238mg) was dropped at -78 ℃, and the mixture was stirred at the same temperature for 30 minutes. After stirring for 15 minutes triethylamine (671μl) was added to the reaction mixture, and the temperature was raised to room temperature. Saturated sodium chloride aqueous solution was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was dried anhydrous magnesium sulfate and concentrated under reduced pressure to give the crude title compound (236mg).
1 H-NMR (400MHz, CDCl 3) δ (ppm): 1.67 (dd, J = 8.0,4.8Hz, 1H), 1.96-2.00 (m, 1H), 2.36 (s, 3H), 2.49-2.55 (m, 1H), 2.59 (s, 3H), 4.19 (d, J = 9.6Hz, 1H), 4.44 (d, J = 10.0Hz, 1H), 6.97-7.04 (m, 1H), 7.14-7.20 (m, 1H), 7.21-7.25 (m, 1H), 7.30 -7.37 (m, 1H), 7.95 (s, 1H), 9.87 (d, J = 3.2Hz, 1H).
(6) (1R, 2S) -2 – {[(2,4- dimethyl-pyrimidin-5-yl) oxy] methyl} -2- (3-fluorophenyl) cyclopropanecarboxylic acid (Prep14-6)Compound Prep14- 5 (18.9g) and 2-methyl-2-butene (26.1ml), sodium dihydrogen phosphate the (9.07g) was dissolved in acetone-water mixed solvent (200ml · 40ml), sodium chlorite ( 6.26g) and I were added little by little. After stirring for 2 hours at room temperature, the reaction solution was concentrated under reduced pressure. The precipitated solid was filtered off, washed with dichloromethane, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel column chromatography (n- heptane: After 1, ethyl acetate: ethyl acetate = 1: 1-0 methanol = 10: 1) to give the title compound (16.2g) was purified by.
1 H-NMR (400MHz, CDCl 3) δ (ppm): 1.55 (dd, J = 8.4,5.6Hz, 1H), 1.76 (t, J = 5.6Hz, 1H), 2 .25 (dd, J = 8.4,6.4Hz, 1H), 2.33 (s, 3H), 2.55 (s, 3H), 4.47 (t, J = 9.6Hz, 1H) , 4.50 (d, J = 9.6Hz, 1H), 6.99 (tdd, J = 8.0,2.4,1.2Hz, 1H), 7.21 (dt, J = 9.6 , 2.4Hz, 1H), 7.26 (td, J = 8.0,1.2Hz, 1H), 7.32 (td, J = 8.0,6.0Hz, 1H), 8.21 ( s, 1H).
Compound Prep14-6 can be prepared directly by the following method from the compound Prep14-4.
Compound Prep14-4 (300mg) and TEMPO (5mol%, 7.74mg) was dissolved in phosphate buffer solution of acetonitrile · pH6.4 (5ml · 5ml), 2N- hydrochloric acid (150μl), sodium chlorite (180mg ) and it was added. After heating to 40 °, 5w% of the hypochlorite solution (2mol%, 26.5μl) were added and stirred for 2 hours. Cooled to room temperature, the reaction mixture was stirred for 5 minutes was added an excess of 2-methyl-2-butene in. The reaction solution was extracted with dichloromethane, the solvent was distilled off under reduced pressure, the residue was purified by silica gel column chromatography (n- heptane: ethyl acetate = 1: 1 to 0: After 1, ethyl acetate: methanol = 9: 1) in was purified to give the title compound (215mg).
Example 95
(1R, 2S) -2 – {[(2,4- dimethyl-pyrimidin-5-yl) oxy] methyl} -2- (3-fluorophenyl) -N- (5- fluoro-2-yl) cyclopropane The synthesis of carboxamide (95)
Acid Prep14-6 a (226mg) was dissolved in dichloromethane (10ml), oxalyl chloride (122μl), and stirred for 1 hour at room temperature was added DMF (a few drops). The reaction mixture was concentrated under reduced pressure to give the crude acid chloride. N in THF (10ml) solution of 2-amino-5-fluoro pyridine (96.1mg), N- diisopropylethylamine (283μl) was added mixture was heated to 60 ℃, the temperature of intact dropwise a THF solution of the crude acid chloride in it was allowed to stir for 1 hour. The reaction mixture was allowed to cool to room temperature and allowed to stir for 1 hour, after which the reaction mixture was concentrated under reduced pressure, partitioned between ethyl acetate and water, the organic layer was separated. The organic layer was dried over anhydrous magnesium sulfate, and the filtrate was concentrated under reduced pressure. The residue was purified by NH- silica gel column chromatography (n- heptane: ethyl acetate = 2: 1) to give diethyl ether to the obtained target compound was added. The precipitated solid was filtered dried to give the title compound (130mg).
1 H-NMR (400MHz, d-DMSO) δ (ppm): 1.46-1.50 (m, 1H), 1.68 (t, J = 6.0Hz, 1H), 2.01 (s, 3H), 2.36 (s, 3H), 2.59-2.63 (m, 1H), 4.27 (d, J = 10.4Hz, 1H), 4.66 (d, J = 10. 4Hz, 1H), 7.06-7.11 (m, 1H), 7.37-7.44 (m, 3H), 7.60-7.65 (m, 1H), 7.85-7. 89 (m, 1H), 8.11 (s, 1H), 8.30 (d, J = 3.2Hz, 1H), 11.20 (brs, 1H)
MS [M + H] + = 411
Synthesis coming…….watch out
References
- Christopher, John A (2014). “Small-molecule antagonists of the orexin receptors”. Pharmaceutical Patent Analyst 3 (6): 625–638.doi:10.4155/ppa.14.46. ISSN 2046-8954.
- Cristoph Boss, Catherine Ross (2015). “Recent Trends in Orexin Research – 2010 to 2015”. ScienceDirect.doi:10.1016/j.bmcl.2015.05.012.
- Boss, Christoph (2014). “Orexin receptor antagonists – a patent review (2010 to August 2014)”. Expert Opinion on Therapeutic Patents 24 (12): 1367–1381.doi:10.1517/13543776.2014.978859. ISSN 1354-3776.
- AdisInsight. “Lemborexant”. Springer. Retrieved 2015-05-23.

External links

| Systematic (IUPAC) name | |
|---|---|
|
(1R,2S)-2-[(2,4-dimethylpyrimidin-5-yl)oxymethyl]-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropane-1-carboxamide
|
|
| Clinical data | |
| Legal status |
|
| Identifiers | |
| CAS Registry Number | 1369764-02-2 |
| ATC code | None |
| PubChem | CID: 56944144 |
| ChemSpider | 34500836 |
| Chemical data | |
| Formula | C22H20F2N4O2 |
| Molecular mass | 410.417 g/mol |







No comments:
Post a Comment