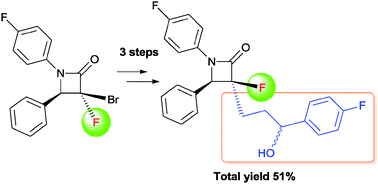
Synthesis of a fluorinated Ezetimibe analogue using radical allylation of [small alpha]-bromo-[small alpha]-fluoro-[small beta]-lactam
New J. Chem., 2015, Advance Article
DOI: 10.1039/C5NJ01969A, Paper
DOI: 10.1039/C5NJ01969A, Paper
Atsushi Tarui, Ayumi Tanaka, Masakazu Ueo, Kazuyuki Sato, Masaaki Omote, Akira Ando
*Corresponding authors
aFaculty of Pharmaceutical Sciences, Setsunan University, 45-1 Nagaotoge-cho, Hirakata, Japan
E-mail: aando@pharm.setsunan.ac.jp
E-mail: aando@pharm.setsunan.ac.jp
http://pubs.rsc.org/en/Content/ArticleLanding/2015/NJ/C5NJ01969A?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FNJ+%28RSC+-+New+J.+Chem.+latest+articles%29#!divAbstract
No comments:
Post a Comment