


cas 920493-71-6 and 898911-09-6
DRL 17822
MW 603.6045, MFC30 H31 F6 N7
Cas 898911-09-6, 1454689-50-9
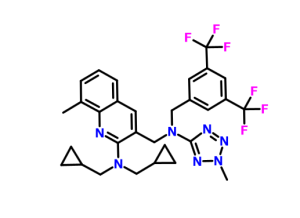
3-([[3,5-Bis(trifluoromethyl)benzyl](2-methyl-2H-tetrazol-5-yl)amino]methyl)-N,N-bis(cyclopropylmethyl)-8-methylquinolin-2-amine
3-Quinolinemethanamine, 2-[bis(cyclopropylmethyl)amino]-N-[[3,5-bis(trifluoromethyl)phenyl]methyl]-8-methyl-N-(2-methyl-2H-tetrazol-5-yl)-
3-(((3,5-bis(trifluoromethyl)benzyl)(2- methyl-2H-tetrazol-5-yl)amino)methyl)-N,N-bis(cyclopropylmethyl)-8- methylquinolin-2-amine
(3-{ [3,5-bis-trifluoromethyl-benzyl)-(2-methyl-2H-tetrazoIe-5-yl)- amino]-methyl}-8-methyl-quinolin-2-yl)-bis-cyclopropylmethyl-amine
Reddy US Therapeutics (Innovator)
Dr. Reddy's Laboratories Ltd.




Treatment of Atherosclerosis Therapy Lipoprotein Disorders,
CETP inhibitor (dyslipidemia/atherosclerosis/cardiovascular diseases), Dr Reddy's
Selective inhibitor of cholesteryl ester transfer protein (CETP)
- 30 Jun 2012Dr Reddy's Laboratories completes a phase II trial in Hypercholesterolaemia in Italy, Poland and Ukraine (NCT01388816)
- 09 Mar 2012Dr Reddy's Laboratories completes enrolment in its phase II trial for Hypercholesterolaemia in Italy, Poland, and Ukraine (NCT01388816)
- 02 Sep 2011Phase-II clinical trials in Hypercholesterolaemia in Ukraine (PO)
Cardiovascular disease is a leading cause of death worldwide. Among cardiovascular disorders, coronary heart disease (CHD) caused by atherosclerosis is the most common cause of morbidity and mortality. Prevention, stabilization and regression of atherosclerotic plaques may have a major impact on reducing the risk of acute coronary events.
LDL-C lowering agents, primarily the statins, are the current mainstay in the pharmacologic management of dyslipidemia. However even with stain use, residual CHD risk from dyslipidemia remains. Epidemiologic and observational studies have shown that HDL-C is also a strong independent predictor of CHD, suggesting that raising HDL-C levels might afford clinical benefit in the reduction of cardiovascular risk.
Presently only niacin is approved by the FDA for HDL-C elevation and can raise HDL-C levels by 20-30%. However its use can be limited by a high incidence of flushing and, less commonly, by elevation of blood glucose and potential hepatic toxicity.
Cholesteryl ester transfer protein (CETP) inhibitors are being explored for their ability to elevate HDL-C. A small molecule CETP inhibitor, torcetrapib, has been demonstrated to elevate HDL-C by 60-100%. However, a large clinical trial (ILLUMINATE) where it increased HDL-C by a mean of 72% compared to baseline was halted as it failed to show benefit. Post-hoc analysis of this study implicated an off-target increase in blood pressure as potentially counteracting any anti-atherosclerotic benefits. Post-hoc subgroup analysis showed that patients in the highest HDL-C quartile had a 57% reduction in the risk of cardiovascular events.
Increased blood pressure appears to be specifically related to torcetrapib as two other small molecule CETP inhibitors, anacetrapib and dalcetrapib, have not shown this in clinical trials and have been well tolerated. DRL-17822 has also not shown elevation of blood pressure in either animals or in normal volunteers.
This study will investigate the efficacy and tolerability of DRL-17822 as dyslipidemia monotherapy in patients with Type II hyperlipidemia.
Hyperlipidemia or an elevation in serum lipids is associated with an increase incidence of cardiovascular disease and atherosclerosis. Primary hyperlipidemia is a term used to describe a defect in lipoprotein metabolism. The lipoproteins commonly affected are low density lipoprotein (LDL) cholesterol, which transports mainly cholesterol, and very low density lipoprotein-cholesterol (VLDL-cholesterol), which transports mainly triglycerides (TG). Most subjects with hyperlipidemia have a defect in LDL metabolism, characterized by raised cholesterol, LDL-C levels, with or without raised triglyceride levels; such subjects are termed hypercholesterolemic (Fredrickson Type II). Familial hypercholesterolemia (FH) is caused by any one of a number of genetically-determined defects in the LDL receptor, which is important for the entry of cholesterol into cells. The condition is characterized by a reduced number of functional LDL receptors, and is therefore associated with raised serum LDL-C levels due to an increase in LDL.

It is reasonably known in the art that the likelihood of cardiovascular disease can be decreased, if the serum lipids, and in particular LDL-C, can be reduced. It is further known that the progression of atherosclerosis can be retarded or the regression of atherosclerosis can be induced if serum lipids can be lowered. In such cases, individuals diagnosed with hyperlipidemia or hypercholesteremia should consider lipid-lowering therapy to retard the progression or induce the regression of atherosclerosis for purposes of reducing their risk of cardiovascular disease, and in particular coronary artery disease.
Cholesteryl ester-transfer protein (CETP) is an important player in metabolism of lipoproteins, such as, for example, a high density lipoprotein (HDL). CETP is a 70 kDa plasma glycoprotein that is physically associated with HDL particles. It facilitates the transport of cholesteryl ester from HDL to apolipoprotein B-containing lipoproteins. This transfer is accompanied by transfer of triglycerides in the opposite direction. Thus, a decrease in CETP activity can result in an increase in the level of HDL cholesterol and a decrease in the level of very low density lipoprotein (VLDL) and low density lipoprotein (LDL). CETP can therefore simultaneously affect the concentrations of pro-atherogenic (for example, LDL) and anti-atherogenic (for example, HDL) lipoproteins.
Several CETP inhibitors are currently in various clinical phases of development for treating various aforementioned disorders. In spite of having various advantages, CETP inhibitors are proven to be difficult to formulate for oral administration. CETP inhibitors are of a highly lipophilic nature and have extremely low solubility in water. Due to their poor solubility, bioavailability of conventional oral compositions is very poor. The lipophilic nature of CETP inhibitors not only leads to low solubility but also tends to poor wettability, further reducing their tendency to be absorbed from the gastrointestinal tract. In addition to the low solubility, CETP inhibitors also tend to have significant, "food effect", where a significant difference in rate and amount of drug absorption is observed when the drug is administered with or without a meal. This "food effect", often complicates the dosing regimen and may require high dosing to achieve the desired therapeutic effect, resulting in potentially unwanted side effects.
Several attempts have been made to improve the solubility of CETP inhibitors, but have generally ended up with limited success. At the outset, most methods aimed at enhancing aqueous concentration and bioavailability of low-solubility drugs only offer moderate improvements. References describing improving the dissolution of poorly soluble drugs include: U.S. Patent Nos. 5,456,923, 5,993,858, 6,057,289, 6,096,338, 6,267,985, 6,280,770, 6,436,430, 6,451,339, 6,531,139, 6,555,558, 6,638,522, 6,962,931 and 7,374,779.
PATENT
WO 2014128564
https://www.google.co.in/patents/WO2014128564A2?cl=en
WO-2014076568
http://www.google.com/patents/WO2014076568A2?cl=en
EXAMPLES
In the following Examples 1-17, various compositions in accordance with the present application were prepared comprising 3-(((3,5-bis(trifluoromethyl)benzyl)(2- methyl-2H-tetrazol-5-yl)amino)methyl)-N,N-bis(cyclopropylmethyl)-8- methylquinolin-2-amine as the CETP inhibitor.:
EXAMPLE 1 :
1. 3-(((3,5-bis(trifluoromethyl)benzyl)(2-methyl-2H-tetrazol-5-yl)amino)methyl)- N,N-bis(cyclopropylmethyl)-8-methylquinolin-2-amineand hydroxypropyl methyl cellulose acetate succinate were mixed together in given solvent mixture to form clear solution.
2. To the solution of step I, Polyoxyl 35 castor oil and talc were added to form a homogenous suspension.
3. The suspension of step 2 was sprayed over inert sugar spheres and dried.
4. The drug layered spheres of step 3 were coated with dispersion made from given seal layer ingredients.
5. The coated spheres of step 4 were formulated further as capsule dosage form.
PATENT
WO 2013046045
https://www.google.co.in/patents/WO2013046045A1?cl=en
PATENT
WO 2013024358
PATENT
WO 2007075194
https://www.google.co.in/patents/WO2007075194A1?cl=en
Syntheis construction






Example 1
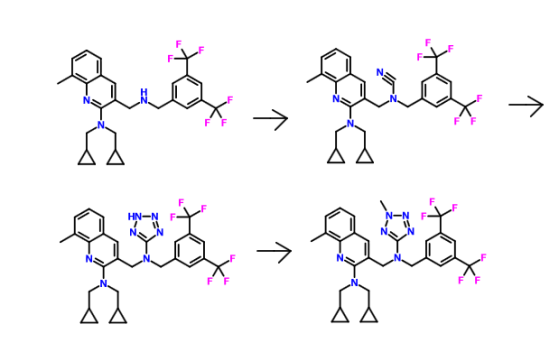
Synthesis of (3-{[3,5-bis trifluoromethyl-benzyl )-(2-cyclopropyImethyI-2H- tetrazole -5-yl)-amino]-methyl-}-8-methyI-quinolme-2-yl)-bis- cyclopropylmethyl-amine Step (i): Synthesis of 2~chloro-8-methyl-quinoline-3-carbaldehyde
DMF (1.22 g, 16.7 mmol) was taken in a flask equipped with a drying tube and POCl3 (7.32 g, 46.7 mmol) was added dropwise with stirring at 0° C. To this solution, TV-o-Tolyl acetamide (1.00 g, 6.7 mmol) was added and the solution was refluxed for 6 h at 90° C. The excess POCl3 was distilled off, water was added to the residue and this was stirred at room temperature for 10 min. The solid was filtered and dried under vacuum..This crude compound was purified over silica gel (100-200 mesh) using 6% ethyl acetate and petroleum ether to give the product as a yellowish solid (yield: 78%). 1H NMR (CDCl3, 200 MHz): δ 10.5 (s, IH)5 8.71 (s, IH), 7.83- 7.79 (m, IH), 7.74- 7.70 (m, IH), 7.56-7.49 (m, IH), 2.79 (s, 3H); m/z (EI-MS): 206 (M+, 100%). Step (ϋ): Synthesis of 2-(bis(cyclopropylmethyl)amino)-8-methylquinoline-3- carbaldehyde:
2-Chloro-8-methyl-quinoline-3-carbaldehyde (.115 g, 0.559 mmol), and potassium carbonate (0.231 g, 1.67 mmol) were put in a 25 mL two necked RB flask. To this, 3 mL of DMF was added followed by dropwise addition of bis- cyclopropylmethyl amine (0.083 g, 0.67 mmol). The reaction mixture was refluxed for 2 h and was cooled to RT. It was then poured on crushed ice (10 mL) and extracted with EtOAc (3 x 10 mL). The organic layer was washed with brine and dried over sodium sulphate. The solvent was evaporated under vacuum to give a yellow colored oil (0.081 g, 50%).
1H NMR (CDCl3, 400 MHz): δ 10.5 (s, IH), 8.71 (s, IH), 7.83- 7.79 (m, IH),
7.74-7.70 (m, IH), 7.56-7.49 (m, IH), 3.55-3.47 (m, 4H), 2.79 (s, 3H), 1.73-1.72
(m, 2H), 1.70-1.46 (m, 4H), 1.20-1.11 (m, 4H); m/z (ES-MS ): 295 (M+H-I5
100%); IR (neat, cm"1): 3385, 2948, 1691.
Step (iii): Synthesis of 3-((3,5-bis(trifluoromethyl)benzylamino)methyl)-N,N- bis(cyclopropylmethyl)-8-methylquinolin-2-amine
2-(Bis(cyclopropylmethyl)amino)-8-methylquinoline-3-carbaldehyde (0.081 g, 0.39 mmol), 3,5-bis-trifluoromethylbenzylamine (0.096 g, 0.39 mmol) and acetic acid (0.047 g, 0.78 mmol) were put in a 25 mL RB flask. To this, 2 rnL of methanol was added and stirred at RT for 15 min. Sodium cyanoborohydri.de (0.075 g, 0.77 mmol) was added portionwise and stirring was continued at RT for another 1 h. Methanol was removed from the reaction mixture under vacuum, water was added to this crude and was extracted with ethyl acetate (3 x 50 mL). The organic layer was washed with saturated NaHCO3 solution, brine and dried over sodium sulphate. The solvent was evaporated and the crude residue was purified by column chromatography over silica gel (100-200 mesh) eluting with 4% ethyl acetate in petroleum ether to give the title amine (0.142 g, yield: 99%). 1R NMR (CDCl3, 400 MHz): δ 7.89-7.86 (m, IH), 7.80 (m, IH), 7.75-7.74 (m, IH), 7.60-7.40 (m, 3H), 7.30-7.26 (m,lH), 4.12 (s, 2H), 3.88 (s, 2H), 3.24-3.22 (m, 4H), 2.72 (s, 3H), 0.99-0.92 (m, 2H), 0.44-0.35 (m, 4H), 0.11-0.05 (m, 4H); m/z (EI-MS ): 522 (M++l, 100%); IR (neat, cm"1): 3357, 2929, 2851.
Step (iv): Synthesis of N-(3,5-bis(trifluoromethyl)benzyl)-N-((2- (bis(cyclopropylmethyl)amino)-8-methylqumolin-3-yl)methyl)cyanamide
To a solution of 3-((3,5~bis(trifluoromethyl)benzylamino)methyl)-N,N- bis(cyclopropylmethyl)-8-methylquinolin-2-amine (0.176 g , 0.33 mmol ), obtained in step (iii) , in MeOH (4 mL) under N2 atmosphere was added sodium bicarbonate (0.056 g, 0.67 mmol ) followed by the addition of cyanogen bromide (0.063 g, 0.60 mmol). The reaction mixture was stirred at RT for 2 h. The solvent was removed under vacuum to give the crude residue which was dissolved in water, extracted with ethyl acetate and dried over sodium sulphate. The solvent was evaporated and concentrated in vacuo to afford N-(3,5-bis(trifluoromethyl)benzyl)- N-((2-(bis(cyclopropylmethyl)amino)-8-methylquinolin-3-yl)methyl)cyanamide (0.118 g, 64%).
1H NMR (CDCl3, 400 MHz ): δ 8.07 (s, IH) , 7.82 (s, IH), 7.70 (s, 2H), 7.56-7.55 (m, IH), 7.50-7.49 (m, IH), 4.49 (s, 2H), 4.23 (s, 2H), 3.17 -3.15 (m, 4H), 2.71 (s, 3H), 0.097-0.085 (m, 2H), 0.405-0.401 (m, 4H), 0.385-0.381 (m, 4H); m/z (ES- MS): 547 (M++l, 100%); IR(KBr ,Cm"1 ) : 2273, 1280.
Step (v): Synthesis of (3-{[(3,5-bistrifluoromethyl-benzyl)-(2H-tetrazol-5-yl)- amino]-methyl}-8-methyl-quinolin-2-yl)-bis-cyclopropylmethyl-amine
7V-(3,5-Bis(tiifluoromethyl)benzyl)-N-((2-(bis(cyclopropylmethyl)amino)- 8-methylqumolin-3-yl)methyl)cyanamide (0.118 g, 0.216 mmol), sodium azide (0.70 g 1.08 mmol) and ammonium chloride (.058 g, 1.08 mmol) were put in a RB flask under N2atmosphere. To this reaction mixture, DMF (2 mL) was added and was refluxed for 1 h. The reaction mixture was cooled to RT and ice was added to this and extracted with ethylacetate (3x10 mL). The combined organic layer was washed with brine, dried over sodium sulphate and then concentrated under vacuum to afford of (3-{[(3,5-bistrifluoromethyl-benzyl)-(2H-tetrazol-5-yl)- amino]-methyl}-8-methyl-quinolin-2-yl)-bis-cyclopropylmethyl-amine as a yellow solid (0.125 g, 99%).
1H NMR (CDCl3, 400 MHz ): δ 7.99 (s, IH) , 7.79 -7.74 (m, 4H ), 7.41-7.40 (m,
IH ), 7.33-7.31 (m, IH), 4.99 (s, 2H), 4.80 (s, 2H), 3.68 (s, 4H), 2.16 (s, IH) 1.56-
1.06 (m, HH); m/z (ES-MS): 578 (M++l, 100%); IR (KBr , cm"1) 3680 , 2922 ,
1660 , 1616.
METHYLATION SHOULD GIVE THE PRODUCT
Scheme 1
WO 2006073973
http://www.google.co.in/patents/WO2006073973A2?cl=en
Example 47
Synthesis of [2-(bis-cycIopropylmethyI-amino)-8-methyl-quinolin-3-ylmethyI]-(3,5- bis-trifluoromethyl-benzyl)-carbamic acid methyl ester
Step (i): Synthesis of bis-cyclopropylmethyl-amine
(i) a. Synthesis of cyclopropanecarboxylic acid cyclopropylmethyl-amide:
Cyclopropyl carboxylic acid (1.0 g, 11.63 mmol) was added to a 50 mL two neck round bottom flask, along with DCM (25 mL). This mixture was cooled to 0° C, EDCI (4.15 g, 13.95 mmol) was added portionwise to the mixture with stirring under nitrogen atmosphere, and the temperature was maintained for 0.5 h. After this time, hydroxybenzotriazole (1.88 g, 13.95 mmol) was added to the 0° C mixture which was stirred for 10 min, then triethylamine (1.7 g, 11.63 mmol) was added, and stirring of the mixture was continued at the same temperature for another 0.5 h. Then, cyclopropylmethylamine (0.825 g, 11.63 mmol) was added, and the reaction was allowed to reach RT, and stirring was continued overnight. The solvent was then removed in vacuo, and the crude residue was purified by passing through a column over 60-120 silica gel, eluting with dichloromethane, to afford the title compound (1.6 g), yield: 87%. 1H NMR (CDCl3, 200 MHz): d 5.75 (br s, NH, D2O exchangeable), 3.17-3.16 (m, 2H), 1.00-0.80 (m, 4H), 0.77-0.67 (m, 2H), 0.56-0.43 (m, 2H), 0.24-0.16 (m, 2H) m/z (CI-MS): 139 (M+, 100%) (i) b. Synthesis of bis-cyclopropylmethyl-amine
To a suspension of lithium aluminum hydride (1.3 g, 9.35mmol) in 10 mL dry ether, a solution of N-cyclopentenoyl-ethylamine (1.7 g, 13.3 mmol) in dry ether (10 mL) was added under a nitrogen atmosphere. This reaction was stirred at RT for 8 h and the reaction mixture was then quenched with saturated sodium sulfate solution, filtered, and the precipitate was washed with diethyl ether. The filtrate was concentrated to afford the title amine (0.8 g), yield: 69%.
1H NMR (CDCl3, 200 MHz): d 5.75 (br s, NH, D2O exchangeable), 3.16-3.09 (m, 2H), 2.50-2.4 (m, 2H), 0.56-0.43 (m, 4H), 0.24-0.21 (m, 3H), 0.21-0.13 (m, 3H) m/z (ES-MS): 139 (M^+14, 100%)
Step (ii): Synthesis of [2-(bis-cyclopropylmethyl-amino)-8-methyl-quinolin-3-ylmethyl]- (S^-bis-trifluoromethyl-benzy^-carbamicacid methyl ester
The title compound was synthesized by using the same procedure as in Example 35, except using o-tolyl acetanilide in step (i) instead of acetanilide and bis- cyclopropylmethyl amine in step (iii), which yielded the desired product as a light yellow, viscous liquid (0.05 g), yield:40%, of purity 98.8% (HPLC: Symmetry Shield RP8, [0.01M KH2PO4: CH3CN], 217 nM, Rt12.719 min).
1H NMR (CDCl3, 400 MHz): d 7.7 (s, IH), 7.68-7.44 (m, 3H), 7.27-7.24 (m, 2H), 4.78- 4.65 (m, 2H), 4.47-4.4 (m, 2H), 3.8 (s, 3H), 3.16-3.14 (d, J=7Hz, 2H), 2.7 (s, 3H), 1.55 (s, 3H), 1.01-0.9(m, IH), 0.38-0.34 (m, 4H), 0.07-0.05 (m, 4H); m/z (CI-MS): 579 (M+, 100%)
Example 57
Synthesis of (3-{ [3,5-bis-trifluoromethyl-benzyl)-(2-methyl-2H-tetrazoIe-5-yl)- amino]-methyl}-8-methyl-quinolin-2-yl)-bis-cyclopropylmethyl-amine
The title compound was prepared as an oil by following the same synthetic procedures as in Example 52, except using {3-[(3,5-bis-trifluoromethyl-benzylamino)- methyl]-8-methyl-quinolin-2-yl}-bis-cyclopropylmethyl-amine in step (i) instead of {3- [(3,5-bis-trifluoromethyl-benzylamino)-methyl]-quinolin-2-yl}-cyclopentylmethyl-ethyl- amine (0.07 g), yield: 52%.
Purity: 95.53% (HPLC: Symmetry Shield RP8, [0.01M KH2PO4: CH3CN], 217 nM, Rt 9.538 min).
IR (neat, cm4) 3079, 2925, 1582;
1H NMR (CDCl3, 400 MHz): d 7.82 (s, IH), 7.69-7.67 (m, 2H), 7.44-7.41 (m, IH), 7.23- 7.2 (m, 3H), 4.91 (s, 2H), 4.65 (s, 2H), 4.21 (s, 3H), 3.29 -3.19 (m, 4H)5 2.71 (s, 3H), 1.01-1.00 (m, 2H), 0.99-0.83 (m, 2H), 0.39-0.34 (m, 3H), 0.08-0.07 (m, 3H). m/z (ES-MS): 604 (M++!, 100%)
Dr. Reddy’s announces start of Phase II study with the CETP inhibitor, DRL-17822 in dyslipidemia patients
Hyderabad, India, September 02, 2011: Dr
Reddy’s Laboratories (NYSE: RDY) announced the initiation of dosing
with DRL-17822 in patients with diagnosis of type II dyslipidaemia.
DRL-17822, is a selective, orally bioavailable inhibitor of cholesteryl
ester transfer protein (CETP), for the treatment and/or prevention of
dyslipidaemia, atherosclerosis and associated cardiovascular disease.
The
current study is being conducted under a CTA in a number of countries
in Europe. The objective of the study is to evaluate the efficacy and
safety of DRL-17822 in patients with Type-II dyslipidemia. This is a
randomized, double blind, placebo controlled, parallel group study in
160 subjects. The primary outcome measure is to assess the elevation in
HDL cholesterol and reduction in LDL cholesterol from baseline to end of
treatment compared to placebo. Three doses (50, 150 & 300 mg) of
DRL-17822 given once daily for 4 weeks will be evaluated during this
study.Three human Phase I studies with DRL-17822 had already been conducted in Europe, where DRL-17822 was shown to be safe and well tolerated. In these studies, the proof of mechanism had been demonstrated by dose-dependent inhibition of plasma CETP activity as well as by significant increase in HDL cholesterol & decrease in LDL cholesterol levels.
Cardiovascular disease is a leading cause of death among men and women worldwide. Among cardiovascular disorders, coronary heart disease (CHD), caused by atherosclerosis is the most common cause of morbidity and mortality. Stabilization and/or regression of atherosclerotic plaques may have a major impact on reducing the risk of acute coronary events. Low-density lipoprotein cholesterol lowering agents, primarily the statins, are the current mainstay in the pharmacological management of dyslipidaemia. However, significant residual cardiovascular risk remains despite use of statins.
Epidemiological and observational studies demonstrate that reduced high density lipoprotein cholesterol levels are a strong, independent predictor of CHD, suggesting that raising HDL cholesterol levels might afford clinical benefit in the reduction of cardiovascular risk. One approach to raise HDL level has been inhibition of CETP activity. Currently it is believed that, raising HDL cholesterol and lowering LDL cholesterol through CETP inhibition would lead to a significant benefit in terms of CHD risk reduction.
Dr. K. Anji Reddy, Founder Chairman, Dr. Reddy’s Laboratories added, “We are committed to delivering products of differentiated value in this area of high global clinical unmet need. We are excited to continue to advance our CETP program and look forward to the data from our Phase II study. This class of therapy could transform the treatment of CHD and DRL 17822 is in a position to be one of the front-running products in the class”.
Disclaimer
This press release includes forward-looking statements, as defined in the U.S. Private Securities Litigation Reform Act of 1995. We have based these forward-looking statements on our current expectations and projections about future events. Such statements involve known and unknown risks, uncertainties and other factors that may cause actual results to differ materially. Such factors include, but are not limited to, changes in local and global economic conditions, our ability to successfully implement our strategy, the market acceptance of and demand for our products, our growth and expansion, technological change and our exposure to market risks. By their nature, these expectations and projections are only estimates and could be materially different from actual results in the future.
About Dr. Reddy’s
Dr. Reddy’s Laboratories Ltd. (NYSE: RDY) is an integrated global pharmaceutical company, committed to providing affordable and innovative medicines for healthier lives. Through its three businesses - Pharmaceutical Services and Active Ingredients, Global Generics and Proprietary Products – Dr. Reddy’s offers a portfolio of products and services including APIs, custom pharmaceutical services, generics, biosimilars, differentiated formulations and NCEs. Therapeutic focus is on gastro-intestinal, cardiovascular, diabetology, oncology, pain management, anti-infective and pediatrics. Major markets include India, USA, Russia and CIS, Germany, UK, Venezuela, S. Africa, Romania, and New Zealand. For more information, log on to: www.drreddys.com
For more information please contact:
Investors and Financial Analysts:Kedar Upadhye at kedaru@drreddys.com / +91-40-66834297
Raghavender R at raghavenderr@drreddys.com / +91-40-49002135
Milan Kalawadia (USA) at mkalawadia@drreddys.com / +1 908-203-4931
Media:
S Rajan at rajans@drreddys.com / +91-40- 49002445
S Rajan at rajans@drreddys.com / +91-40- 49002445
| WO2005011634A1 * | Jul 21, 2004 | Feb 10, 2005 | William John Curatolo | Dosage forms providing controlled release of cholesteryl ester transfer protein inhibitors and immediate release of hmg-coa reductase inhibitors |
| WO2006073973A2 * | Dec 28, 2005 | Jul 13, 2006 | Reddy Us Therapeutics Inc | Novel benzylamine derivatives as cetp inhibitors |
| WO2006129167A1 * | May 22, 2006 | Dec 7, 2006 | Pfizer Prod Inc | PHARMACEUTICAL COMPOSITIONS OF CHOLESTERYL ESTER TRANSFER PROTEIN INHIBITORS AND HMG-CoA REDUCTASE INHIBITORS |
| WO2007128568A1 * | May 8, 2007 | Nov 15, 2007 | Novartis Ag | Bicyclic derivatives as cetp inhibitors |
| EP0298666A2 * | Jul 1, 1988 | Jan 11, 1989 | American Home Products Corporation | Spray dried ibuprofen compositions |
| EP1741424A2 * | Jul 27, 1998 | Jan 10, 2007 | Pfizer Products Inc. | Solid pharmaceutical dispersions with enhanced bioavailabilty |
| US5456923 | Dec 23, 1993 | Oct 10, 1995 | Nippon Shinyaku Company, Limited | Method of manufacturing solid dispersion |
| US5474989 | Mar 1, 1994 | Dec 12, 1995 | Kurita Water Industries, Ltd. | Drug composition |
| US5985326 | May 30, 1996 | Nov 16, 1999 | Icos Corporation | Method of producing a solid dispersion of a poorly water soluble drug |
| US6350786 | Sep 7, 1999 | Feb 26, 2002 | Hoffmann-La Roche Inc. | Stable complexes of poorly soluble compounds in ionic polymers |
| US6548555 | Jan 31, 2000 | Apr 15, 2003 | Pfizer Inc | Basic drug compositions with enhanced bioavailability |
| US6638522 | Dec 10, 1999 | Oct 28, 2003 | Pharmasolutions, Inc. | Microemulsion concentrate composition of cyclosporin |
| US6730679 | Mar 20, 1997 | May 4, 2004 | Smithkline Beecham Corporation | Pharmaceutical formulations |
| US7008640 | Jul 16, 2001 | Mar 7, 2006 | Yamanouchi Pharmaceutical Co., Ltd. | Pharmaceutical composition for oral use with improved absorption |
| US7034013 | Mar 19, 2002 | Apr 25, 2006 | Cydex, Inc. | Formulations containing propofol and a sulfoalkyl ether cyclodextrin |
| US7037528 | Jun 5, 2001 | May 2, 2006 | Baxter International Inc. | Microprecipitation method for preparing submicron suspensions |
| US7078057 | Dec 19, 2000 | Jul 18, 2006 | Kerkhof Nicholas J | Process for producing nanometer particles by fluid bed spray-drying |
| US7081255 | Aug 14, 2002 | Jul 25, 2006 | Janssen Pharmaceutica, N.V. | Antifungal compositions with improved bioavailability |
| US8030359 | Feb 9, 2007 | Oct 4, 2011 | Merck Sharp & Dohme Corp. | Polymer formulations of CETP inhibitors |
| US20060178514 | Dec 28, 2005 | Aug 10, 2006 | Anima Baruah | Novel benzylamine derivatives as CETP inhibitors |
| Reference | ||
|---|---|---|
| 1 | * | EINFAL T ET AL: "Methods of amorphization and investigation of the amorphous state", ACTA PHARMACEUTICA 20130901 CROATIAN PHARMACEUTICAL SOCIETY DEU, vol. 63, no. 3, 1 September 2013 (2013-09-01), pages 305-334, XP002721717, ISSN: 1330-0075 |
| 2 | * | KAI TOSHIYA ET AL: "Oral absorption improvement of poorly soluble drug using solid dispersion technique", CHEMICAL AND PHARMACEUTICAL BULLETIN (TOKYO), vol. 44, no. 3, 1996, pages 568-571, XP002721716, ISSN: 0009-2363 |
| 3 | * | KIM TAE-WAN ET AL: "Characterization of dual layered pellets for sustained release of poorly water-soluble drug", CHEMICAL & PHARMACEUTICAL BULLETIN (TOKYO), vol. 55, no. 7, July 2007 (2007-07), pages 975-979, XP002721715, ISSN: 0009-2363 |
| 4 | * | KIM TAE-WAN ET AL: "Modified release of coated sugar spheres using drug-containing polymeric dispersions.", ARCHIVES OF PHARMACAL RESEARCH JAN 2007, vol. 30, no. 1, January 2007 (2007-01), pages 124-130, XP002721714, ISSN: 0253-6269 |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| WO2014128564A2 * | Feb 21, 2014 | Aug 28, 2014 | Dr. Reddy's Laboratories Ltd. | Pharmaceutical compositions of cetp inhibitors |
http://circ.ahajournals.org/cgi/content/meeting_abstract/122/21_MeetingAbstracts/A13981

////////
Cn1nc(nn1)N(Cc2cc5cccc(C)c5nc2N(CC3CC3)CC4CC4)Cc6cc(cc(c6)C(F)(F)F)C(F)(F)F






No comments:
Post a Comment