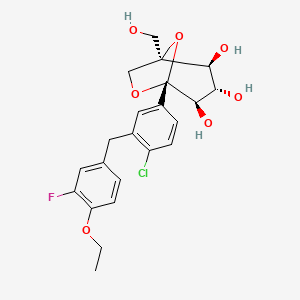
Henagliflozin, SHR-3824 ,
CAS 1623804-44-3
C22-H24-Cl-F-O7
454.8756
PHASE 2 for the treatment of type 2 diabetes
HengRui (Originator)
| Jiangsu Hengrui Medicine Co Ltd |
In April 2016, Jiangsu Hengrui Medicine is developing henagliflozin (phase 2 clinical trial), a sodium-glucose cotransporter-2 (SGLT-2) inhibitor, for treating type 2 diabetes.
SGLT1 and SGLT2 inhibitors, useful for treating eg diabetes.
Henagliflozin proline is in phase II clinical trials by Jiangsu Hengrui (江苏恒瑞) for the treatment of type 2 diabetes.
1,6-dehydrated-1-C{4-chloro-3-[(3-fluoro-4-ethoxyphenyl)methyl]phenyl}-5-C-(hydroxymethyl)-β-L-idopyranose L-proline
(1 ^ 2345-5- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -1- (hydroxymethyl) 6,8 - alcohol dioxide
(1R,2S,3S,4R,5R)-5-[4-chloro-3-[(4-ethoxy-3-fluorophenyl)methyl]phenyl]-1-(hydroxymethyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol
Shanghai Hengrui Pharmaceutical Co., Ltd., 上海恒瑞医药有限公司, Jiangsu Hengrui Medicine Co., Ltd., 江苏恒瑞医药股份有限公司, Less «
- 01 May 2015 Jiangsu HengRui Medicine Co. initiates enrolment in a phase I drug interaction trial in volunteers in China (NCT02500485)
- 12 Feb 2015 Jiangsu HengRui Medicine plans a phase I trial for Type-2 diabetes mellitus in China (NCT02366377)
- 01 Feb 2015 Jiangsu HengRui Medicine initiates enrolment in a phase I trial for Type-2 diabetes mellitus in China (NCT02366351)

PATENT
WO-2016050134
With
the improvement of socio-economic development and living standards,
worldwide rapid growth of diabetes, diabetes is usually divided into two
kinds of diabetes type Ⅰ and type Ⅱ diabetes, more than 90% of type Ⅱ
diabetes. Species has been listed diabetes drugs a lot, but so far, no
drugs which can single-handedly blood glucose levels in patients with
type Ⅱ diabetes in the long-term target range. In recent years, in-depth
study of the pathogenesis of diabetes, for the treatment of type Ⅱ
diabetes provide more and more ways, and sodium - glucose cotransporter 2
(sodium-glucose transporter 2, SGLT-2) inhibitors found for treatment
of diabetes provides another new idea. SGLT-2 inhibitors in the
treatment mechanism of inhibition of SGLT-2 activity by selective to
lower blood sugar. Select the SGLT-2 as a target, partly because of its
absolute weight of glucose absorption, and partly because it is only
expressed in the kidney. The current study also found that the mechanism
of SGLT-2 does not depend on the degree of abnormal function of β cells
or insulin resistance, its effect is not as severe failure or insulin
resistance and β-cell function decline.Therefore, it is reasonable that
the SGLT-2 inhibitors for the treatment of type Ⅱ diabetes currently has
good prospects.
WO2012019496
discloses SGLT-2 inhibitor of the following formula, and its chemical
name is 1,6-anhydro -1-C- {4- chloro-3 - [(3-fluoro-4-ethoxyphenyl)
methyl] phenyl} -5-C- (hydroxymethyl) -β-L- idose pyranose.
However,
direct 1,6-anhydro -1-C- {4- chloro-3 - [(3-fluoro-4-ethoxyphenyl)
methyl] phenyl} -5-C- (hydroxymethyl) - β-L- idose pyranose as a
pharmaceutically active ingredient is not realistic, because a lower
melting point (83 ℃), having a hygroscopicity, poor development of the
form, therefore, to develop it into a stable form of the compound having
the transformation very important.
Example 1
Take
(1.0g, 2.2mmol) 1,6- dehydration -1-C- {4- chloro-3 -
[(3-fluoro-4-ethoxyphenyl) methyl] phenyl} -5-C- ( hydroxymethyl) -β-L-
Aidoo pyranose (prepared by the method disclosed in WO2012019496), in
7.20g ethanol addition was completed, stirring to dissolve. Was
added at room temperature L- proline (0.2786g, 2.42mmol, 1.1eq), the
addition was completed, the reaction was warmed at reflux for 10min, the
reaction solution was clear, hot filtered and the filtrate was stirred
to room temperature, there is a lot of white solid precipitated ,
allowed to stand overnight, filtered, and dried, to give the formula
(I), compound as a white solid 1.14 g, yield 88%. X- ray diffraction
spectrum of the crystalline sample is shown in Figure 1. The
crystallization at about 5.41 (16.33) 7.69 (11.49), 10.22 (8.65) 12.04
(7.35), 12.46 (7.10), 14.42 (6.14), 17.30 (5.12), 18.79 (4.72), 19.38
(4.58), 20.24 (4.38), 22.73 (3.91), 24.58 (3.62), 27.55 (3.24), 28.82
(3.10) and 31.03 (2.88) at the characteristic peaks. DSC spectrum shown
in Figure 2, has a melting endothermic peak 111.20 ℃, this is defined as
a Form A polymorph.
PATENT
WO2012019496
https://www.google.com/patents/WO2012019496A1?cl=en
Example 4
(1 ^ 2345-5- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -1- (hydroxymethyl) 6,8 - alcohol dioxide
first step
1-ethoxy-2-fluoro - benzene
A mixture of 2-fluoro-phenol 4a (6.7 g, 60 mmol) was dissolved in 66 mL of acetone, was added iodoethane (6.3 mL,
78 mmol) and potassium carbonate (12.4 g, 90 mmol), at reflux in an oil bath for 5 hours. The reaction solution was concentrated under reduced pressure, was added 100 mL of ethyl acetate and 60 mL of water, separated, the aqueous phase was extracted with ethyl acetate (30 mLx2), the organic phases combined, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure, to give the title product 1-ethoxy-2-fluoro - benzene 4b (6.9 g, red oil). yield: 82.1%.
MS m / z (ESI): 280.2 [2M + 1]
The second step
(5-bromo-2-chloro - phenyl) - (4-ethoxy-3-fluoro-phenyl) - methanone A mixture of 5-bromo-2-chloro - benzoyl chloride 2a (12.4 g, 48.8 mmol) was dissolved a 100 mL of dichloromethane was added 1-ethoxy-2-fluoro - benzene 4b (6.84 g, 48.8 mmol), cooled to 0 ° C, was added portionwise aluminum (5.86 g, 44 mmol) chloride, 16 h. Was added dropwise under ice-cooling to the reaction mixture 20 mL of 2 M HCl solution, separated, the aqueous phase was extracted with 30 mL of dichloromethane, and the combined organic phase was dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to give the title The product (5-bromo-2-chloro - phenyl) - (4-ethoxy-3-fluoro-phenyl) - methanone 4c (12.7 g, yellow solid), yield: 72.6%.
MS m / z (ESI): 358.9 [M + l] Step
(5 - bromo-2-chloro - phenyl) - (4-ethoxy-3-fluoro-phenyl) - methanol (5-Bromo-2-chloro - phenyl) - (4-ethoxy -3 - fluoro - phenyl) -methanone 4c (12.7 g, 35.5 mmol) was dissolved in methanol and a 100 mL of tetrahydrofuran (ν: ν = 1: 1) mixed solvent, under an ice bath was added portionwise sodium borohydride (2.68 g, 70 mmol), and reacted at room temperature for 30 minutes. Add 15 mL of acetone, the reaction solution was concentrated under reduced pressure, 150 mL of ethyl acetate was added to dissolve the residue, washed with saturated sodium chloride solution (50 mLx2). The combined organic phase was dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure The filtrate, to give the title product (5-bromo-2-chloro - phenyl) - (4-ethoxy-3-fluoro-phenyl) - methanol 4d (12.7 g, orange oil), was used directly without isolation next reaction.
the fourth step
4 - [(5-bromo-2-chloro-phenyl) - methyl] Small-ethoxy-2-fluoro - benzene (5-bromo-2-chloro - phenyl) - (4-ethoxy -3 - fluoro - phenyl) methanol 4d (12.7 g, 35.3 mmol) was dissolved in a 100 mL of dichloromethane was added triethylsilane (16.9 mL, 106 mmol), was added dropwise boron trifluoride etherate (8.95 mL, 70.6 mmol ), for 3 hours. Was added 50 mL of saturated sodium bicarbonate solution, separated, the aqueous phase was extracted with ethyl acetate (100 mLx2), the organic phases combined, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure, purified by silica gel column chromatography to elute B surfactant system resulting residue was purified to give the title product 4 - [(5-bromo-2-chloro - phenyl) methyl] -1-ethoxy-2-fluoro - benzene 4e (10 g, as a pale yellow oil ) yield: 82.4%.
1H NMR (400 MHz, CDC1 3 ): δ 7.33-7.27 (m, 3H), 6.95-6.90 (m, 3H), 4.14 (q, 2H), 4.01 (s, 2H), 1.49 (t, 3H)
the fifth step
(2 3R, 4S, 5 ^ 6R) -2- [4- chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -6- (hydroxymethyl) - 2-methoxy - tetrahydro-pyran-3,4,5-triol
4 - [(5-bromo-2-chloro - phenyl) methyl] -1-ethoxy-2-fluoro - benzene 4e (7.36 g, 21.4 mmol) was dissolved in 30 mL of tetrahydrofuran, cooled to -78 ° C, was added dropwise a solution of n-butyllithium in hexane (10.27 mL, 25.7 mmol), at -78 ° C to react 1 hour, a solution of 20 mL (3R, 4S, 5R, 6R) -3,4,5 - tris (trimethylsilyloxy) -6- (trimethylsilyloxy) tetrahydropyran-2-one 2f (llg, 23.6 mmol) in tetrahydrofuran at -78 ° C under reaction 2 h, 2.8 mL of methanesulfonic acid and 71 mL of methanol, the reaction at room temperature for 16 hours. Was added 100 mL of saturated sodium carbonate solution, the reaction solution was concentrated under reduced pressure, to the residue was added 50 mL of saturated sodium chloride solution, extracted with ethyl acetate (100 mLx3), organic phases were combined, dried over anhydrous magnesium sulfate, filtered, The filtrate was concentrated under reduced pressure, purified by silica gel column chromatography with eluent systems resulting A residue was purified to give the title product (2 3R, 4S, 5 6R) -2- [4- chloro-3 - [(4-ethoxyphenyl 3-fluoro-phenyl) - methyl] phenyl] -6- (hydroxymethyl) -2-methoxy - tetrahydro-pyran-3,4,5-triol 4f (5.7 g, white solid ) yield: 58.3%.
1H NMR (400 MHz, CD 3 OD): δ 7.56 (s, 1H), 7.48 (dd, 1H), 7.37 (dd, 1H), 6.95-6.87 (m, 3H), 4.08-4.07 (m, 4H) , 3.91 (m, 1H), 3.93-3.73 (m, 2H), 3.56-3.53 (m, 1H), 3.45-3.43 (m, 1H), 3.30 (s, 2H), 3.08 (s, 3H), 1.35 (t, 3H)
The sixth step
(2 3R, 4S, 5 6R) -6- [(tert-butyl (dimethyl) silyl) oxymethyl] -2- [4-chloro-3 - [(4-ethoxy-3-fluoro - phenyl) methyl] phenyl] -2-methoxy - tetrahydro-pyran-3,4,5-triol the (2 3R, 4S, 5 6R) -2- [4- chloro-3- [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -6- (hydroxymethyl) -2-methoxy - 4f tetrahydropyran-3,4,5-triol (5.7 g, 12.5 mmol) was dissolved in 50 mL of pyridine, followed by adding tert-butyldimethylsilyl chloride (2.26 g, 15 mmol) and 4-dimethylaminopyridine (305 mg, 2.5 mmol), for 16 hours. The reaction solution was concentrated under reduced pressure, was added 200 mL of ethyl acetate, washed with a saturated copper sulfate solution (50 mLx3). The combined organic phase was dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to give the title product (2 3R, 4S, 5 6R) -6- [(tert-butyl (dimethyl) silyl) oxymethyl] -2- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -2-methoxy - tetrahydro-pyran-3,4,5-triol 4g (7.14 g, colorless oil), without isolation directly used for the next reaction.
Seventh Step
[[(2R, 3R, 4S, 5R, 6 ^ -3,4,5- tris-benzyloxy-6- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl yl] phenyl] -6-methoxy - tetrahydropyran-2-yl] methoxy] - tert-butyl - dimethyl-silane (2 3R, 4S, 5 6R) -6- [(tert butyl (dimethyl) silyl) oxymethyl] -2- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -2-methoxy yl - tetrahydro-pyran-3,4,5-triol 4g (7.14 g, 12.5 mmol) was dissolved in 100 mL N, N- dimethylformamide was added 60% sodium hydride under ice-cooling (2.5 g , 62.5 mmol), and reacted at room temperature for 40 minutes completed the opening force, was added benzyl bromide (7.5 mL, 62.5 mmol), reaction of 16 hours. 20 mL of methanol, the reaction solution was concentrated under reduced pressure, was added 200 mL of ethyl acetate and 50 mL of water to dissolve the residue, separated, the aqueous phase was extracted with ethyl acetate (50 mL), the organic phase was washed with water (50 mL), washed with saturated sodium chloride solution (50 mL), the combined organic phase was dried over anhydrous magnesium sulfate , filtered, and the filtrate was concentrated under reduced pressure to give the title product [[(2R, 3R, 4S, 5R, 6 ^ -3,4,5- tris-benzyloxy-6- [4-chloro-3 - [(4- ethoxy-3-fluoro-phenyl) - methyl] phenyl] -6-methoxy - tetrahydropyran-2-yl] methoxy] - tert-butyl - dimethylsilane 4h (10.5 g , yellow oil) yield: 99.8%.
Step Eight
[(2R, 3R, 4S, 5R, 6 -3,4,5- tris-benzyloxy-6- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -6-methoxy - tetrahydropyran-2-yl] methanol
The [[(2R, 3R, 4S, 5R, 6 -3,4,5- tris-benzyloxy-6- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl yl] phenyl] -6-methoxy - tetrahydropyran-2-yl] methoxy] - tert-butyl - dimethylsilane 4h (10.52 g, 12.5 mmol) was dissolved in 50 mL of methanol dropwise add acetyl chloride CO.13 mL, 1.9 mmol), for 1 hour. The reaction solution was concentrated under reduced pressure, purified by silica gel column chromatography with eluent systems B resultant residue was purified to give the title product [(2R, 3R, 4S, 5R, 6 -3,4,5- tris-benzyloxy--6 - [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -6-methoxy - tetrahydropyran-2-yl] methanol 4i (7.6 g , yellow oil yield: 83.6%.
Step Nine
(2 ^ 3456 3,4,5-tris-benzyloxy-6- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] - 6-methoxy - tetrahydropyran-2-carbaldehyde
Oxalyl chloride (1.17 mL, 13.6 mmol) was dissolved in 20 mL of dichloromethane, cooled to -78 ° C, were added dropwise 20 mL of dimethyl sulfoxide (1.56 mL, 21.9 mmol) in methylene chloride and 50 mL [(2R, 3R, 4S, 5R, 6 -3,4,5- tris-benzyloxy-6- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -6-methoxy - tetrahydropyran-2-yl] methanol 4i (7.6 g, 10.45 mmol) in methylene chloride, and reacted at -78 ° C for 30 min, triethylamine (7.25 mL, 52.3 mmol), 2 hours at room temperature was added 50 mL 1 M HCl solution, separated, the organic phase was washed with saturated sodium chloride solution (50 mL x 2), the aqueous phase was extracted with dichloromethane (50 mL), the combined organic phase was dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to give the title product (2 ^ 3456 3,4,5-tris-benzyloxy-6- [4-chloro-3 - [(4 - ethoxy-3-fluoro-phenyl) - methyl] phenyl] -6-methoxy - tetrahydropyran-2-carbaldehyde 4j (7.58 g, colorless oil), was used directly without isolation next reaction.
The tenth step
(2S, 3 4S, 5R, 6 -3,4,5- tris-benzyloxy-6- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl ] -2- (hydroxymethyl) -6-methoxy - tetrahydropyran-2-carbaldehyde
The (23456 3,4,5-tris-benzyloxy-6- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] - 6-methoxy - tetrahydropyran-2-carbaldehyde 4j (7.6 g, 10.45 mmol) was dissolved in 80 mL 1,4- dioxane, followed by adding 15.8 mL 37% aqueous formaldehyde and sodium hydroxide solution (31.35 mL, 31.35 mmol), reacted at 70 ° C for 16 h. Add 50 mL of saturated sodium chloride solution, extracted with ethyl acetate (50 mLx4), the organic phase was washed with saturated sodium bicarbonate solution (50 mL), washed with saturated sodium chloride solution (50 mL), the combined organic phase was dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to give the title product (23,456 benzyloxy-3,4,5-tris - 6- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -2- (hydroxymethyl) -6-methoxy - tetrahydropyran - 2- formaldehyde 4k (7.9g, as a colorless oil), without isolation directly used for the next reaction.
Step Eleven
[(3 4S, 5R, 6 -3,4,5- tris-benzyloxy-6- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] 2- (hydroxymethyl) -6-methoxy - tetrahydropyran-2-yl] methanol
The (23456 3,4,5-tris-benzyloxy-6- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] - 2- (hydroxymethyl) -6-methoxy - tetrahydropyran-2-carbaldehyde 4k (7.9 g, 10.45 mmol) was dissolved in 50 mL of tetrahydrofuran and methanol (v: v = 2: 3) mixed solvent , was added sodium borohydride (794 mg, 20.9 mmol), for 30 minutes. Add a small amount of acetone, the reaction solution was concentrated under reduced pressure, purified by silica gel column chromatography with eluent systems resulting A residue was purified to give the title product, 5R, 6 -3,4,5-tris-benzyloxy-6- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -2- (hydroxymethyl ) -6-methoxy - tetrahydropyran-2-yl] methanol 4m (l.ll g, colorless oil). yield: 14.1%.
Step Twelve
[(12345 ^ -2,3,4-tris-benzyloxy-5- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] 6,8-dioxa-bicyclo [3.2.1] octane-1-yl] methanol
The [(3S, 4S, 5R, 6 -3,4,5- tris-benzyloxy-6- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] benzene yl] -2- (hydroxymethyl) -6-methoxy - tetrahydropyran-2-yl] methanol 4m (l.ll g, 1.46 mmol) was dissolved in 20 mL of dichloromethane, cooled to -10 ° C, was added trifluoroacetic acid (0.23 mL, 3 mmol), and reacted at room temperature for 2 hours. 20 mL of saturated sodium bicarbonate solution, separated, the aqueous phase was extracted with dichloromethane (20 mL> <2), and the combined organic phase was dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure, purified by silica gel column chromatography with eluent systems B resultant residue was purified to give the title product [(1 2 3 4R, 5 -2,3,4- tris-benzyloxy-5- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] 6,8-dioxa-bicyclo [3.2.1] octane-1-yl] methanol 4nC830 mg, colorless oil). yield: 78.3%.
MS m / z (ESI): 742.3 [M + 18]
Thirteenth Step
(12345-5- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -1- (hydroxymethyl) -6,8 dioxa-bicyclo [3.2.1] octane-2,3,4-triol
The [(1 2 3 4R, 5S) -2,3,4- tris-benzyloxy-5- [4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] benzene yl] -6,8-dioxa-bicyclo [3.2.1] octane-1-yl] methanol 4n (830 mg, 1.14 mmol) was dissolved in 20 mL of tetrahydrofuran and methanol (v: v = l: l) the a mixed solvent of o-dichlorobenzene was added (1.3 mL, 1 1.4 mmol) and Pd / C (500 mg, 10%), purged with hydrogen three times, the reaction for 3 hours. The reaction solution was filtered, rinsed with a small amount of ethyl acetate, the filtrate was concentrated under reduced pressure, purified by silica gel column chromatography with eluent systems resulting A residue was purified to give the title product (1S, 2 3S, 4R, 5 -5- [ 4-chloro-3 - [(4-ethoxy-3-fluoro-phenyl) - methyl] phenyl] -1- (hydroxymethyl) -6,8-dioxa-bicyclo [3.2.1] octane-2,3,4-triol 4 (420 mg, white solid), yield: 81.0% MS m / z (ESI):. 472.2 [m + 18]
1H NMR (400 MHz, CD 3 OD): δ 7.47 (s, 1H), 7.42-7.35 (m, 2H), 6.95-6.87 (m, 3H), 4.16-4.14 (m, 1H), 4.06-4.02 ( m, 4H), 3.85-3.70 (m, 2H), 3.67-3.54 (m, 4H), 1.37 (t, 3H)
////////Henagliflozin, SHR-3824 , PHASE 2, type 2 diabete, UNII-21P2M98388, 21P2M98388, SHR 3824, SHR3824,
CCOc1ccc(cc1F)Cc2cc(ccc2Cl)[C@]34[C@@H]([C@H]([C@@H]([C@](O3)(CO4)CO)O)O)O


No comments:
Post a Comment