TD-1607
Phase I
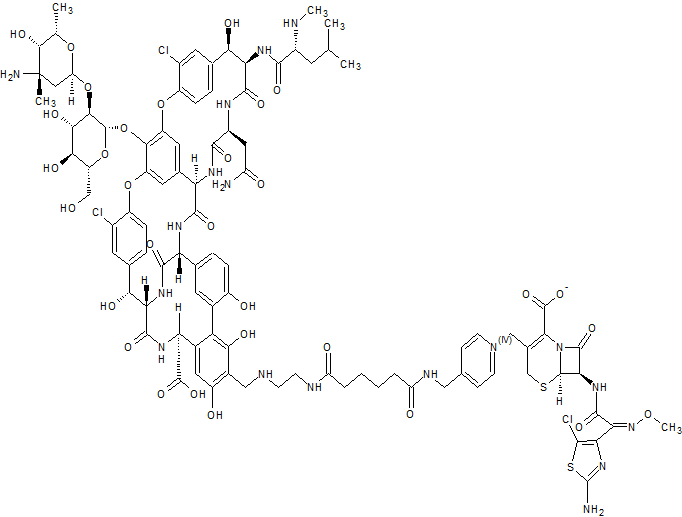
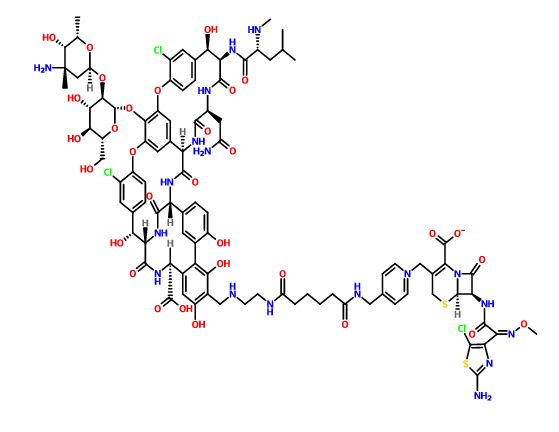
A glycopeptide-cephalosporin heterodimer potentially for the treatment of gram-positive bacterial infection.
CAS No. 827040-07-3
C95 H109 Cl3 N18 O31 S2,
Molecular Weight, 2169.47
Vancomycin, 29-[[[2-[[6-[[[1-[[(6R,7R)-7-[[(2Z)-2-(2-amino-5-chloro-4-thiazolyl)-2-(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]pyridinium-4-yl]methyl]amino]-1,6-dioxohexyl]amino]ethyl]amino]methyl]-, inner salt
Vancomycin, 29-[[[2-[[6-[[[1-[[(6R,7R)-7-[[(2Z)-(2-amino-5-chloro-4-thiazolyl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]pyridinium-4-yl]methyl]amino]-1,6-dioxohexyl]amino]ethyl]amino]methyl]-, inner salt
- Originator Theravance
- Developer Theravance Biopharma
- Class Antibacterials; Cephalosporins; Glycopeptides
- Mechanism of Action Cell wall inhibitors
- Phase I Gram-positive infections
Most Recent Events
- 21 Apr 2016 Phase I development is ongoing in USA
- 01 Jul 2014 Theravance completes a phase I trial in Healthy volunteers in in USA (NCT01949103)
- 02 Jun 2014 Theravance Biopharma is formed as a spin-off of Theravance
Company Theravance Biopharma Inc. Description Glycopeptide cephalosporin heterodimer antibiotic Molecular Target Mechanism of Action Therapeutic Modality Small molecule: Combination Latest Stage of Development Phase I Standard Indication Gram-negative bacterial infection Indication Details Treat Gram-positive bacterial infections


PATENT
WO 2005005436
The present invention provides novel cross-linked glycopeptide - cephalosporin compounds that are useful as antibiotics. The compounds of this invention have a unique chemical structure in which a glycopeptide group is covalently linked to a pyridinium moiety of a cephalosporin group. Among other properties, compounds of this invention have been found to possess surprising and unexpected potency against Gram-positive bacteria including methicillin-resistant Staphylococci aureus (MRSA). Accordingly, in one aspect, the invention provides a compound of formula I:
////////Theravance Biopharma, TD 1607, phase 1, glycopeptide-cephalosporin heterodimer , gram-positive bacterial infection

No comments:
Post a Comment