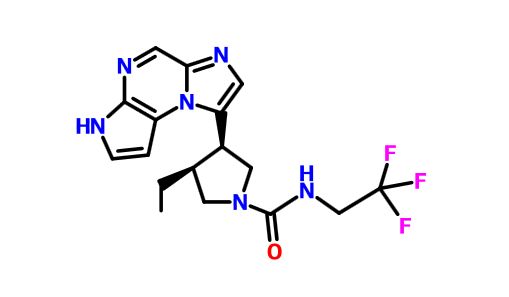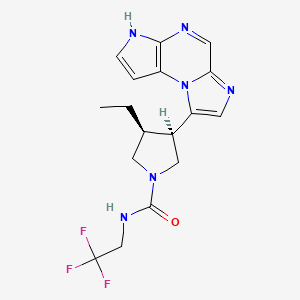
ABT 494
(-)-(3S,4R) cis form
CAS 1310726-60-3 FREE FORM
| MF | C17H19F3N6O |
|---|---|
| MW | 380.36757 g/mol |
Tartrate form
C17 H19 F3 N6 O . C4 H6 O6 . 4 H2 O ………….CAS 1607431-21-9
1-Pyrrolidinecarboxamide, 3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)-, (3S,4R)-, (2R,3R)-2,3-dihydroxybutanedioate, hydrate (1:1:4)
FREE FORM
(3s,,4R)-3-ethyl-4-(3H-imidazo[l,2-fl]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine- l-carboxamide.
(35,,4R)-3-ethyl-4-(3H- imidazo[l,2-fl]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine- l-carboxamide,
(cis,)-3-ethyl-4-(3H-imidazo[l,2-fl]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-l-carboxamide
1-Pyrrolidinecarboxamide, 3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)-, (3S,4R)-
rel-(-)-(3S,4R)-3-Ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide
pharmaceutically acceptable salts thereof, stereoisomers thereof, and isomers thereof, is provided in U.S. Patent No. 8,426,411,
Abbott Laboratories ABBOTT ……INNOVATOR
AbbVie, a global biopharmaceutical company, today announced the start of a large Phase 3 clinical trial program to study the use of ABT-494, an investigational, once-daily, oral selective JAK1 inhibitor for the treatment of rheumatoid arthritis (RA). This program will include adult patients with inadequate responses (IR) to conventional or biologic disease-modifying antirheumatic drugs (DMARDs), as well as methotrexate-naive patients.
PATENT
WO2015061665
The synthesis of the compounds of the invention, including (35,,4R)-3-ethyl-4-(3H-imidazo[l,2-fl]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine- l-carboxamide, pharmaceutically acceptable salts thereof, stereoisomers thereof, and isomers thereof, is provided in U.S. Patent No. 8,426,411, the entire content of which is incorporated herein by reference.
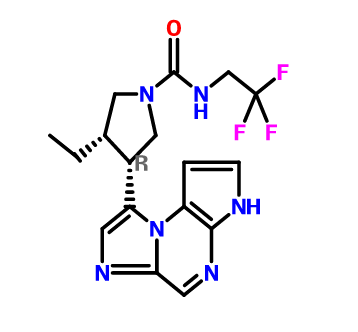
For example, (3lS,,4R)-3-ethyl-4-(3H-imidazo[l,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-l-carboxamide can be synthesized according to the following scheme:
N-Alkylation using alkyl halide, a-haloketone or oc-haloamide
A round bottom flask is charged with a base such as NaH (60% dispersion in mineral oil), K2CO3, or CS2CO3 (preferably NaH (60% dispersion in mineral oil), 0.9-1.5 equiv., preferably 0.95 equiv.) and an organic solvent (such as N, N-dimethylformamide (DMF), dichloromethane (DCM), 1,4-dioxane, or N-methyl-2-pyrrolidone (NMP), preferably DMF). The mixture is cooled to about -10 °C to ambient temperature (preferably about 0°C) and a solution of an appropriately substituted amine (preferably 1 equiv.) in an organic solvent (such as DMF) is added. Alternatively, the base may be added portionwise to a solution of the amine and an organic solvent at about 0°C to ambient temperature. The reaction mixture is stirred for about 5-90 min (preferably about 15-30 min) at about -10°C to ambient temperature (preferably about 0°C) followed by the addition of an alkyl halide, a-haloketone, or cc-haloamide (1-2 equiv., preferably 1.2 equiv.). Alternatively, a solution of an amine and a base in an organic solvent may be added to a solution of an alkyl halide, α-haloketone, or a-haloamide in an organic solvent at about 0°C. The reaction mixture is stirred at about -10°C to ambient temperature (preferably ambient temperature) for about 0.5-24 h (preferably about 1 h). Optionally, the organic solvent may be removed under reduced pressure.
Optionally, the reaction mixture or residue may be diluted with water, aqueous NH4CI, or aqueous NaHC03. If a precipitate forms the solid may be optionally collected via vacuum filtration to give the target compound. Alternatively, an organic solvent (such as ethyl acetate (EtOAc) or DCM) is added to the aqueous mixture and the layers are separated. The aqueous layer may optionally be extracted further with an organic solvent (such as EtOAc and/or DCM). The combined organic layers are optionally washed with additional aqueous solutions such as brine, dried over anhydrous Na2S04 or MgS04, filtered, and concentrated to dryness under reduced pressure.
The procedure above is illustrated below in the preparation of ie/t-butyl 2-amino-2-oxoethyl(5-tosyl-5H-pyrrolo[3,2-b]pyrazin-2-yl)carbamate from ie/t-butyl (5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-yl)carbamate.
To a solution of iert-butyl 5-tosyl-5H-pyrrolo[3,2-b]pyrazin-2-ylcarbamate (1.00 g, 2.57 mmol, Example #3 Step E) and DMF (13 mL) under nitrogen at about 0 °C was added NaH (60% dispersion in mineral oil, 0.113 g, 2.83 mmol) in one portion. After about 30 min, 2-bromoacetamide (0.391 g, 2.83 mmol) was added in one portion. After about 30 min, the ice bath was removed and the solution was stirred at ambient temperature for about 2 h. Saturated aqueous NH4Cl/water (1: 1, 100 mL) was added. After stirring for about 10 min, the mixture was filtered using water to wash the filter cake. The aqueous phase was extracted with EtOAc (50 mL). The filter cake was dissolved in EtOAc and added to the organic layer. The organic layer was dried over Na2S04, filtered, and concentrated under reduced pressure. The material was purified by silica gel chromatography eluting with a gradient of 20-100% EtOAc/heptane to give tert-butyl 2-amino-2-oxoethyl(5-tosyl-5H-pyrrolo[3,2-b]pyrazin-2-yljcarbamate (0.980 g, 82%): LC/MS (Table 1, Method n) Rt = 0.70 min; MS m/z 446 (M+H)+.
Similar reaction condition can also be used to synthesize benzyl 3-ethyl-4-(2-((5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-yl)amino)acetyl)pyrrolidine-l-carboxylate from iert-butyl (5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-yl)carbamate and benzyl 3-(2-bromoacetyl)-4-ethylpyrrolidine- 1 -carboxylate.
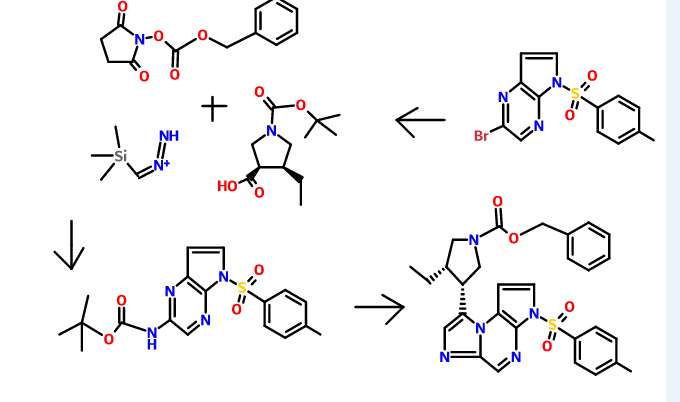
Cyclization of a ketone using a dithiaphosphetane reagent (e.g., synthesizing (3S,4R)-benzyl 3-ethyl-4-(3-tosyl-3H-imidazo[l,2-a]pyrrolo[2,3-e]pyrazin-8-yl)pyrrolidine-l-carboxylate from benzyl 3-ethyl-4-(2-((5-tosyl-5H-pyrrolo[2,3-Z>]pyrazin-2-yl)amino)acetyl)pyrrolidine-l-carboxylate)
To a solution of a ketone (preferably 1 equiv.) in an organic solvent such as tetrahydrofuran (THF) or 1,4-dioxane (preferably 1,4-dioxane) is added a thiolating reagent such as Lawesson’s reagent or Belleau’s reagent (2,4-bis(4-phenoxyphenyl)-l,3-dithia-2,4-diphosphetane-2,4-disulfide) (0.5-2.0 equiv., preferably Lawesson’s reagent, 0.5-0.6 equiv.). The reaction is heated at about 30°C to 120°C (preferably about 60-70°C) for about 0.5-10 h (preferably about 1-2 h). Optionally, additional thiolating reagent (0.5-2.0 equiv., preferably 0.5-0.6 equiv.) can be added to the reaction mixture and heating can be continued for about 0.5-10 h (preferably about 1-2 h). The reaction mixture is concentrated under reduced pressure.
Preparation of 8-((ds)-4-ethylpyrrolidin-3-yl)-3-tosyl-3H-imidazo[l,2-a]pyrrolo[2,3-e]pyrazine from (3S,4R)-benzyl 3-ethyl-4-(3-tosyl-3H-imidazo[l,2-a]pyrrolo[2,3-e]pyrazin-8-yl)pyrrolidine-l-carboxylate
To a solution of (cis)-benzyl 3-ethyl-4-(3-tosyl-3H-imidazo[l,2-a]pyrrolo[2,3-e]pyrazin-8-yl)pyrrolidine-l-carboxylate (0.838 g, 1.541 mmol) is added a solution of HBr (2.50 mL, 15.19 mmol, 33% in acetic acid). The reaction mixture is stirred at ambient temperature for about 1 h. The reaction is diluted with diethyl ether or Et20 (50 mL) and water (20 mL). The layers are stirred for about 3 min and the organic layer is decanted then the procedure is repeated 5 times. The aqueous layer is cooled to about 0°C and is basified with saturated aqueous NaHC03solution (10 mL) to about pH 7. The aqueous layer is extracted with EtOAc (3 x 50 mL), combined, and dried over anhydrous Na2S04, filtered and concentrated to give a brown solid. The solid is dissolved in DCM (50 mL) and washed with water (3 x 20 mL), dried over anhydrous Na2S04, filtered and concentrated to afford 8-((cis)-4-ethylpyrrolidin-3-yl)-3-tosyl-3H-imidazo[l,2-a]pyrrolo[2,3-e]pyrazine (0.453, 61%) as a brown residue: LC/MS (Table 1, Method a) Rt = 1.73 min; MS m/r. 410 (M+H)+.
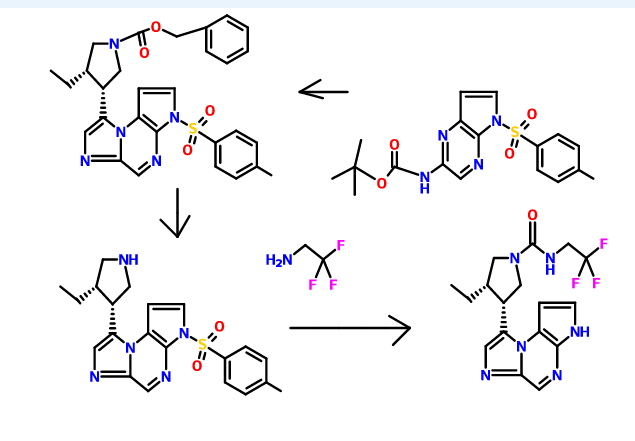
Hydrolysis of a sulfonamide (e.g., 8-((3R,4S)-4-ethylpyrrolidin-3-yl)-3-tosyl-3H-imidazo[l,2-a]pyrrolo[2,3-e]pyrazine to 8-((3R,4S)-4-ethylpyrrolidin-3-yl)-3H-imidazo[l,2-a]pyrrolo[2,3-e]pyrazine)
To a flask containing a sulfonamide, for example, a sulfonyl-protected pyrrole, (preferably 1 equiv.) in an organic solvent (such as 1,4-dioxane, methanol (MeOH), or THF/MeOH, preferably 1,4-dioxane) is added an aqueous base (such as aqueous Na2C03 or aqueous NaOH, 1-30 equiv., preferably 2-3 equiv. for aqueous NaOH, preferably 15-20 equiv. for aqueous Na2C03). The mixture is stirred at about 25-100 °C (preferably about 60 °C) for about 1-72 h (preferably about 1-16 h). In cases where the reaction does not proceed to completion as monitored by TLC, LC/MS, or HPLC, additional aqueous base (such as aqueous Na2C03, 10-20 equiv., preferably 10 equiv. or aqueous NaOH, 1-5 equiv., preferably 1-2 equiv.) and/or a cosolvent (such as ethanol (EtOH)) is added. The reaction is continued at about 25-100°C (preferably about 60°C) for about 0.25-3 h (preferably about 1-2 h). In any case where an additional base labile group is present (for example, an ester a
trifluoromethyl, or a cyano group), this group may also be hydrolyzed. The reaction is worked up using one of the following methods. Method 1. The organic solvent is optionally removed under reduced pressure and the aqueous solution is neutralized with the addition of a suitable aqueous acid (such as aqueous HC1). A suitable organic solvent (such as EtOAc or DCM) and water are added, the layers are separated, and the organic solution is dried over anhydrous Na2S04 or MgS04, filtered, and concentrated to dryness under reduced pressure to give the target compound. Method 2. The organic solvent is optionally removed under reduced pressure, a suitable organic solvent (such as EtOAc or DCM) and water are added, the layers are separated, and the organic solution is dried over anhydrous Na2S04 or MgS04, filtered, and concentrated to dryness under reduced pressure to give the target compound. Method 3. The reaction mixture is concentrated under reduced pressure and directly purified by one of the subsequent methods.
Formation of a urea using CDI or thiocarbonyldiimidazole, respectively (e.g., from 8-((3R,45)-4-ethylpyrrolidin-3-yl)-3H-imidazo[l,2-a]pyrrolo[2,3-e]pyrazine to (35,4R)-3-ethyl-4-(3H-imidazo[l,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-l-carboxamide)
To a solution or slurry of an amine or amine salt (1-3 equiv., preferably 1-2 equiv.) in an organic solvent such as DCM, THF, or DMF (preferably DMF) at about 20 – 80 °C (preferably about 65 °C) is optionally added an organic base, such as triethylamine (TEA), N,N-diisopropylethylamine (DIEA), pyridine (preferably TEA) (1-10 equiv., preferably 1-5 equiv.) followed by CDI or 1,1 ‘-thiocarbonyldiimidazole (0.5-2 equiv., preferably 1 equiv.). After about 0.5-24 h (preferably about 1-3 h), a second amine or amine salt (1-10 equiv., preferably 1-3 equiv.) is added neat or as a solution or slurry in an organic solvent such as DCM, THF, or DMF (preferably DMF). The reaction is held at about 20 – 80 °C (preferably about 65 °C ) for about 2 – 24 h (preferably about 3 h). If the reaction mixture is heated, it is cooled to ambient temperature. The reaction mixture is partitioned between an organic solvent (such as EtOAc, DCM or 1,4-dioxane) and an aqueous base (such as saturated aqueous NaHC03 or saturated aqueous Na2C03, preferably saturated aqueous NaHC03). Optionally, the reaction mixture is concentrated under reduced pressure and the residue is partitioned as above. In either case, the aqueous layer is then optionally extracted with additional organic solvent such as EtOAc or DCM. The combined organic layers may optionally be washed with brine and concentrated in vacuo or dried over anhydrous Na2S04 or MgS04 and then decanted or filtered prior to concentrating under reduced pressure to give the target compound. Optionally, the reaction mixture is concentrated under reduced pressure and the residue is directly purified.
Chiral preparative HPLC purification
Chiral purification is performed using Varian 218 LC pumps, a Varian CVM 500 with
switching valves and heaters for automatic solvent, column and temperature control and a Varian 701 Fraction collector. Detection methods include a Varian 210 variable wavelength detector, an in-line polarimeter (PDR-chiral advanced laser polarimeter, model ALP2002) used to measure qualitative optical rotation (+/-) and an evaporative light scattering detector (ELSD) (a PS-ELS 2100 (Polymer Laboratories)) using a 100: 1 split flow. ELSD settings are as follows: evaporator: 46 °C, nebulizer: 24 °C and gas flow: 1.1 SLM. The absolute stereochemistry of the purified compounds was assigned arbitrarily and is drawn as such. Compounds of the invention where the absolute stereochemistry has been determined by the use of a commercially available enantiomerically pure starting material, or a stereochemically defined intermediate, or X-ray diffraction are denoted by an asterisk after the example number.
(ci5,)-3-ethyl-4-(3H-imidazo[l,2-fl]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-l-carboxamide isolated using the above method has an Rt min of 1.52, and m/z ESI+ (M+H)+ of 381.
The starting materials and intermediates of the above synthesis scheme may be obtained using the following schemes:
Preparation of starting material of l-(tert-butoxycarbonyl)-4-ethylpyrrolidine-3-carboxylic acid
Step A: ethyl pent-2-ynoate to (Z)-ethyl pent-2-enoate
To a slurry of Lindlar catalyst (0.844 g, 0.396 mmol) in THF (100 mL) and pyridine (10.00 mL) is added ethyl pent-2-ynoate (5.22 mL, 39.6 mmol). The reaction mixture is sparged with hydrogen for about 10 min and an atmosphere of hydrogen is maintained via balloon. After about 15 h the reaction mixture is filtered through a pad of Celite®, diluted with Et20 (30 mL) and washed with saturated aqueous CuS04 (40 mL), followed by water (40 mL). The organic layer is separated, dried over anhydrous MgS04, filtered, and concentrated in vacuo to provide crude (Z)-ethyl pent-2-enoate (5 g, 98%). 1H NMR (DMSO-d6) δ 1.05 (t, 3H), 1.28 (t, 3H), 2.65 (m, 2H), 4.18 (q, 2 H), 5.72 (m, 1H), 6.21 (m, 1H).
Step B: (ds)-ethyl l-benzyl-4-ethylpyrrolidine-3-carboxylate (from (Z)-ethyl pent-2-enoate and N-benzyl-l-methoxy-N-((trimethylsilyl)methyl)methanamine)
To a solution of N-benzyl-l-methoxy-N-((trimethylsilyl)methyl)methanamine (9.98 mL, 39.0 mmol) and (Z)-ethyl pent-2-enoate (5 g, 39.0 mmol) in DCM (50 mL) is added trifluoroacetic acid (TFA) (0.030 mL, 0.390 mmol) at RT. After about 2 days, the reaction mixture is concentrated in vacuo to provide crude (cis)-ethyl 1 -benzyl-4-ethylpyrrolidine-3- carboxylate (9.8 g, 96%) as an oil. LC/MS (Table 1, Method a) Rt = 1.62 min; MS m/z: 262 (M+H)+.
Step C: ethyl l-benzyl-4-ethylpyrrolidine-3-carboxylate to (ds)-ethyl 4-ethylpyrrolidine-3-carboxylate
A Parr shaker is charged with PdOH2 on carbon (2.243 g, 3.19 mmol) and (cis)-et yl l-benzyl-4-ethylpyrrolidine-3-carboxylate (16.7 g, 63.9 mmol) followed by EtOH (100 mL). The reaction mixture is degassed and purged with hydrogen gas and shaken on the parr shaker at 60 psi for about 4 days at ambient temperature. The reaction mixture is degassed and purged with nitrogen. The suspension is filtered through a pad of Celite® washing with EtOH (~ 900 mL). The solvent is removed under reduced pressure to afford (cis)-ethyl 4-ethylpyrrolidine-3 -carboxylate (8.69 g, 79%) as an oil: LC/MS (Table 1, Method a) Rt = 1.11 min; MS m/z: 172 (M+H)+.
Step D: (ds)-ethyl 4-ethylpyrrolidine-3-carboxylate to (ds)-l-(tert-butoxycarbonyl)-4-ethylpyrrolidine-3-carboxylic acid
To a flask charged with (cis)-et yl 4-ethylpyrrolidine-3-carboxylate (8.69g, 50.7 mmol) is added aqueous HCl (6N, 130 mL, 782 mmol). The solution is heated at about 75°C for about 12 h. aqueous HCl (6N, 100 mL, 599 mmol) is added and stirred at about 80 °C for about 20 h. Aqueous HCl (6N, 100 mL, 599 mmol) is added and continued stirring at about 80 °C for about 20 h. The reaction mixture is cooled to ambient temperature and the solvent is removed under reduced pressure. 1,4-Dioxane (275 mL) and water (50 mL) are added followed by portionwise addition of Na2C03 (13.5 g, 127 mmol). Di-ie/t-butyl dicarbonate (13.3 g, 60.9 mmol) is added and the reaction mixture is stirred at ambient temperature for about 16 h. The solid is filtered and washed with EtOAc (250 mL). The aqueous layer is acidified with aqueous HCl (IN) to about pH 3-4. The layers are partitioned and the aqueous layer is extracted with EtOAc (3 x 100 mL). The combined organic layers are dried over anhydrous Na2S04, filtered and removed under reduced pressure. As the organic layer is almost fully concentrated (~ 10 mL remaining), a solid precipitated. Heptane (30 mL) is added and the solid is filtered washing with heptane to afford (cis)-l-(tert-butoxycarbonyl)-4-ethylpyrrolidine-3-carboxylic acid (3.9 g, 32%) as an off white solid as product: LC/MS (Table 1, Method c) Rt = 0.57 min; MS m/z: 242 (M-H)~.
Synthesis of Intermediate benzyl 3-(2-bromoacetyl)-4-ethylpyrrolidine-l-carboxylate
Acidic cleavage of a Boc-protected amine (e.g., l-(tert-butoxycarbonyl)-4-ethylpyrrolidine-3-carboxylic acid to 4-ethylpyrrolidine-3-carboxylic acid
hydrochloride)
To a solution of a Boc-protected amine (preferably 1 equiv.) in an organic solvent (such as DCM, 1,4-dioxane, or MeOH) is added TFA or HC1 (preferably 4 N HC1 in 1,4-dioxane, 2-35 equiv., preferably 2-15 equiv.). The reaction is stirred at about 20-100 °C (preferably ambient temperature to about 60 °C) for about 1-24 h (preferably about 1-6 h). In any case where an additional acid labile group is present (for example, a t-butyl ester), this group may also be cleaved during the reaction. Optionally, additional TFA or HC1
(preferably 4 N HC1 in 1,4-dioxane solution, 2-35 equiv., preferably 2-15 equiv.) may be added to the reaction mixture in cases where the reaction does not proceed to completion as monitored by TLC, LC/MS, or HPLC. Once the reaction has proceeded to an acceptable level, the reaction mixture can be concentrated in vacuo to provide the amine as a salt.
Alternatively, the reaction may be partitioned between an organic solvent (such as EtOAc, DCM or 1,4-dioxane) and an aqueous base (such as saturated aqueous NaHC03 or saturated aqueous Na2C03, preferably saturated aqueous NaHC03). The aqueous layer can be optionally extracted with additional organic solvent such as EtOAc or DCM. The combined organic layers may optionally be washed with brine, dried over anhydrous Na2S04 or MgS04, then decanted or filtered, prior to concentrating under reduced pressure to give the target compound.
Cbz-protection of an amine (e.g., 4-ethylpyrrolidine-3-carboxylic acid hydrochloride to l-((benzyloxy)carbonyl)-4-ethylpyrrolidine-3-carboxylic acid)
A solution of an amine or an amine salt (preferably 1 equiv.) and a base (for example, Na2C03 or NaOH, 1-3 equiv., preferably Na2C03, 1.6 equiv.) in water or aqueous organic solvent (for example, water / 1,4-dioxane or water / acetonitrile (MeCN), preferably water/ 1,4-dioxane) is stirred at ambient temperature for about 1-10 min (preferably 5 min). A solution of benzyl 2,5-dioxopyrrolidin-l-yl carbonate (1-2 equiv., preferably 1.0 equiv.) in an organic solvent such as 1,4-dioxane or MeCN is added to the reaction. The reaction is stirred at ambient temperature for about 8-144 h (preferably about 72 h). Optionally, the reaction mixture is concentrated under reduced pressure. The resulting aqueous solution is diluted with an organic solvent (such as EtOAc or DCM). The organic extracts are optionally washed with water and/or brine, dried over anhydrous Na2S04 or MgS04, filtered or decanted, and concentrated under reduced pressure. Alternatively, the resulting aqueous solution is acidified by adding an acid such as aqueous NH4C1 or HC1 and is then extracted with an organic solvent (such as EtOAc or DCM).
Formation of a bromomethyl ketone from an acid (e.g., l-((benzyloxy)carbonyl)-4-ethylpyrrolidine-3-carboxylic acid to benzyl 3-(2-bromoacetyl)-4-ethylpyrrolidine-l-carboxylate)
To a solution of a carboxylic acid (preferably 1 equiv.) in an organic solvent (DCM or 1,2-dichloroethane (DCE), preferably DCM) is slowly added oxalyl chloride (1.2-3.0 equiv., preferably 2.2 equiv.) followed by dropwise addition of DMF (0.01-0.20 equiv., preferably about 0.15 equiv.). The reaction is stirred at about 0-40 °C (preferably ambient temperature) for about 3-24 h (preferably about 14 h) before it is concentrated under reduced pressure to a constant weight to give the crude acid chloride. A solution of a crude acid chloride
(preferably 1 equiv.) in an organic solvent (such as THF, MeCN, Et20, or THF/MeCN, preferably THF/MeCN) is added to trimethylsilyldiazomethane (2.0 M in Et20) or diazomethane solution in Et20 (prepared from DIAZALD® according to Aldrich protocol or J. Chromatogr. Sci. 1991, 29:8) (2-10 equiv., preferably 3.5 equiv. of
trimethylsilyldiazomethane) at about -20-20 °C (preferably about 0 °C) in a suitable organic solvent such as THF, MeCN, Et20, or THF/MeCN (preferably THF/MeCN). The reaction mixture is stirred for about 0.5-5 h (preferably about 3 h) at about -20-20 °C (preferably about 0 °C) before the dropwise addition of 48% aqueous HBr (5-40 equiv., preferably about 10 equiv.). After about 0-30 min, (preferably about 5 min) the reaction mixture can be concentrated to dryness to give the desired product, neutralized by a dropwise addition of saturated aqueous NaHC03 or is optionally washed with brine after optional addition of an organic solvent (such as EtOAc or DCM, preferably EtOAc). In cases where the reaction mixture is subjected to an aqueous work-up, the organic layer is dried over anhydrous Na2S04 or MgS04 (preferably MgS04), filtered, and concentrated under reduced pressure.
Synthesis of Intermediate tert-butyl (5-tosyl-5H-pyrrolo[2,3-Z>]pyrazin-2-yl)carbamate
Step A: 3,5-dibromopyrazin-2-amine to 5-bromo-3-((trimethylsilyl)ethynyl)pyrazin-2-amine
To a solution of 3,5-dibromopyrazin-2-amine (125 g, 494 mmol), TEA (207.0 mL, 1483 mmol), and copper (I) iodide (0.941 g, 4.94 mmol) in THF (1255 mL) is added
PdCl2(PPh3)2 (3.47 g, 4.94 mmol). The reaction mixture is cooled at about -5-0°C and a solution of (trimethylsilyl)acetylene (65.0 mL, 470 mmol) in THF (157 mL) is added dropwise over about 15 min. The reaction mixture is stirred at about -5-0°C for about 1.5 h and then allowed to warm to room temperature (RT) overnight. The reaction mixture is then filtered through a CELITE® pad and washed with THF until no further product eluted. The filtrate is concentrated under reduced pressure to give a brown-orange solid. The solid is triturated and sonicated with warm petroleum ether (b.p. 30-60°C, 400 mL), cooled to RT, collected, washed with petroleum ether (b.p. 30-60°C; 2 x 60 mL), and dried to give 5-bmmo-3-((trimethylsilyl)ethynyl)pyrazin-2-amine (124 g, 93%, 93% purity) as a brown solid: LC/MS (Table 1, Method b) Rt = 2.51 min; MS m/z: 270, 272 (M+H)+.
Step B: 5-bromo-3-((trimethylsilyl)ethynyl)pyrazin-2-amine to 2-bromo-5-tosyl-5H-pyrrolo[2,3-Z>]pyrazine
To a solution of 5-bromo-3-((trimethylsilyl)ethynyl)pyrazin-2-amine (3.00g, 11.1 mmol) in DMF (60 mL) at about 0 °C is added NaH (60% dispersion in mineral oil, 0.577g, 14.4 mmol) in three portions. After about 15 min, p-toluenesulfonyl chloride (2.75g, 14.4 mmol) is added and the reaction is allowed to warm slowly to ambient temperature. After about 16 h, the reaction mixture is poured onto ice-cold water (120 mL) and the precipitate is collected by vacuum filtration. The crude solid is dissolved in DCM (15 mL) and purified by silica gel chromatography eluting with DCM to give 2-bromo-5-tosyl-5H-pyrrolo[2,3-bjpyrazine (2.16 g, 52%): LC/MS (Table 1, Method c) Rt = 1.58 min; MS m/z: 352, 354 (M+H)+.
Step C: 2-bromo-5-tosyl-5H-pyrrolo[2,3-b]pyrazine to methyl 5-tosyl-5H-pyrrolo[2,3-Z>]pyrazine-2-carboxylate
CO is bubbled into an orange solution of 2-bromo-5-tosyl-5H-pyrrolo[2,3-b]pyrazine (50. Og, 142 mmol) in DMF (2.50 L) within a 5 L round bottom flask for about 2 min.
Bis(triphenylphosphine)-palladium(II) dichloride (9.96g, 14.2 mmol), TEA (59 mL, 423 mmol) and MeOH (173.0 mL, 4259 mmol) are added and the flask is fitted with a balloon of CO. The mixture is heated at about 95°C under an atmosphere of CO (1 atmosphere). After stirring overnight, the reaction mixture is cooled to ambient temperature overnight and poured into ice water (3.2 L). The mixture is stirred for about 10 min and the precipitate is collected by filtration, while washing with water, and dried for 1 h. The crude material is dissolved in DCM, separated from residual water, dried over anhydrous MgS04, filtered, added silica gel, and concentrated under reduced pressure to prepare for chromatography. The crude material is purified by silica gel column chromatography eluting with 0-5% MeOH in DCM to yield methyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazine-2-carboxylate with 5 mol% DCM as an excipient (40.7 g, 86%, 93% purity): LC/MS (Table 1, Method a) Rt = 2.35 min;
MS m/z 332 (M+H)+.
Step D: methyl 5-tosyl-5H-pyrrolo[2,3-Z>]pyrazine-2-carboxylate to 5-tosyl-5H-pyrrolo[2,3-/>]pyrazine-2-carboxylic acid
HC1 (6 N aqueous, 714 mL) is added to a yellow solution of methyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazine-2-carboxylate (17.8g, 53.6 mmol) in 1,4-dioxane (715 mL) within a 2 L round bottom flask, and the mixture is heated at about 60°C for about 16 h. The reaction mixture is cooled to ambient temperature. The organic solvent is removed under reduced pressure and the precipitate is collected, washed with water, and dried to yield 5-tosyl-5H-pyrrolo[2,3-b]pyrazine-2-carboxylic acid (14.4 g, 85%) as a yellow solid: LC/MS (Table 1, Method a) Rt = 1.63 min; MS m/z 316 (Μ-Η)“.
Step E: 5-tosyl-5H-pyrrolo[2,3-b]pyrazine-2-carboxylic acid to tert-butyl 5-tosyl-5H-pyrrolo[2,3-Z>]pyrazin-2-ylcarbamate
In a 500 mL round bottom flask, 5-tosyl-5H-pyrrolo[2,3-b]pyrazine-2-carboxylic acid (14.4 g, 45.3 mmol), diphenylphosphoryl azide (9.78 mL, 45.3 mmol) and TEA (13.9 mL, 100 mmol) in ie/t-butanol (i-BuOH) (200 mL) are added to give an orange suspension. The mixture is heated at about 70°C for about 16 h, cooled to ambient temperature and the insoluble material is removed by filtration. The solvent is removed under reduced pressure and the crude material is purified by silica gel column chromatography eluting with 25-60% EtOAc in heptane to yield tert-butyl 5-tosyl-5H-pyrrolo[2,3-b]pyrazin-2-ylcarbamate (9.75 g, 54%) as an off-white solid: LC/MS (Table 1, Method a) Rt = 2.79 min; MS m/z 389 (M+H)+.
PATENT
WO2011068881


PATENT
Preparation #F.1.1: 8-((cis)-4-ethylpyrrolidin-3-yl)-3-tosyl-3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazine
- To a solution of (cis)-benzyl 3-ethyl-4-(3-tosyl-3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)pyrrolidine-1-carboxylate (0.838 g, 1.541 mmol, prepared using E from Example #36 Step D with TFA, N, R, S.1 with Example #3 Step E, and T with Lawesson’s reagent) was added a solution of HBr (2.50 mL, 15.19 mmol, 33% in acetic acid). The reaction mixture was stirred at ambient temperature for about 1 h. The reaction was diluted with Et2O (50 mL) and water (20 mL). The layers were stirred for about 3 min and the organic layer was decanted then the procedure was repeated 5 times. The aqueous layer was cooled to about 0° C. was basified with saturated aqueous NaHCO3solution (10 mL) to about pH 7. The aqueous layer was extracted with EtOAc (3×50 mL), combined, and dried over anhydrous Na2SO4, filtered and concd to give a brown solid. The solid was dissolved in DCM (50 mL) and washed with water (3×20 mL), dried over anhydrous Na2SO4, filtered and coned to afford 8-((cis)-4-ethylpyrrolidin-3-yl)-3-tosyl-3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazine (0.453, 61%) as a brown residue: LC/MS (Table 1, Method a) Rt=1.73 min; MS m/z: 410 (M+H)+.
SEE…………..1-((cis)-4-ethylpyrrolidin-3-yl)-6-tosyl-6H-pyrrolo[2,3-e][1,2,4]triazolo[4,3-a]pyrazine (0.250 g, 0.609 mmol, Example #36, step F)
/////
c21cnc4c(n1c(cn2)[C@@H]3[C@@H](CN(C3)C(=O)NCC(F)(F)F)CC)ccn4
OR
CC[C@@H]1CN(C[C@@H]1c4cnc3cnc2nccc2n34)C(=O)NCC(F)(F)F