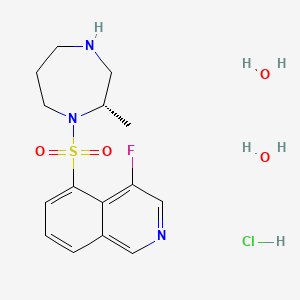
Ripasudil hydrochloride hydrate
4-fluoro-5-[[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl]isoquinoline;dihydrate;hydrochloride
4-Fluoro-5-[2(S)-methylperhydro-1,4-diazepin-1-ylsulfonyl]isoquinoline hydrochloride dihydrate
cas 223645-67-8 FREE
| M.Wt | 395.88 | OR C15H18FN3O2S·HCl·2H2O | |
|---|---|---|---|
| Formula | C15H23ClFN3O4S | ||
| CAS No | 887375-67-9 .HCL 2 H2O |
016TTR32QF, K 115
LAUNCHED 2014 Kowa
JAPAN 2014-09-26, Glanatec

Ripasudil
- Molecular FormulaC15H18FN3O2S
- Average mass323.386
CAS 223645-67-8
4-fluoro-5-[[(2S)-2
| Company | D. Western Therapeutics Institute Inc. |
| Description | Selective rho kinase inhibitor |
| Molecular Target | Rho kinase |
| Mechanism of Action | Rho kinase inhibitor |
SEE
PAPER
HETEROCYCLES, Vol. 83, No. 8, 2011, pg 1771-1781.
Paper | Regular issue | Vol 83, No. 8, 2011, pp.1771-1781
Published online: 24th May, 2011
*Pharmaceutical Division, Tokyo New Drug Research Laboratories, Kowa Co., Ltd., 2-17-43, Noguchicho, Higashimurayama, Tokyo 189-0022, Japan
Published online: 24th May, 2011
DOI: 10.3987/COM-11-12230
■ A Practical Synthesis of Novel Rho-Kinase Inhibitor, (S)-4-Fluoro-5-(2-methyl-1,4-diazepan-1-ylsulfonyl)isoquinoline
Noriaki Gomi, Tadaaki Ohgiya, Kimiyuki Shibuya,* Jyunji Katsuyama, Masayuki Masumoto, and Hitoshi Sakai*Pharmaceutical Division, Tokyo New Drug Research Laboratories, Kowa Co., Ltd., 2-17-43, Noguchicho, Higashimurayama, Tokyo 189-0022, Japan
Abstract
A practical synthesis of novel Rho-kinase inhibitor, (S)-4-fluoro-5-(2-methyl-1,4-diazepan-1-ylsulfonyl)isoquinoline hydrochloride dihydrate (1) was achieved in a pilot-scale production. We have demonstrated the regioselective chlorosulfonylation of 4-fluoroisoquinoline in an one-pot reaction to afford 4-fluoroisoquinoline-5-sulfonyl chloride and the asymmetric construction of the (S)-2-methyl-1,4-diazepane moiety as key steps.
White crystalline solid.: mp 258-259 °C (decomp);
[]20D –8.82 (c1.00, H2O);
IR (KCl) 3406, 2983, 2763, 1588, 1324, 1146, 1129 cm-1; 1H-NMR (DMSO-d6) δ: 1.20 (3H, d,J = 6.6 Hz), 1.98-2.07 (2H, m), 3.06-3.16 (1H, m), 3.22-3.31 (2H, m), 3.35 (4H, s), 3.44 (1H, dd, J = 14.1,4.4 Hz), 3.59-3.74 (2H, m), 4.37-4.47 (1H, m), 7.93 (1H, t, J = 7.8 Hz), 8.32 (1H, d, J = 7.8 Hz), 8.54-8.60(1H, m), 8.72 (1H, d, J = 4.9 Hz), 9.39 (1H, s), 9.51 (2H, br s);
13C-NMR (DMSO-d6) δ: 16.6, 26.8, 42.9,45.5, 50.3, 50.9, 120.9 (J = 12.4 Hz), 127.5, 130.7 (J = 1.7 Hz), 132.2, 132.5 (J = 27.3 Hz), 133.2 (J = 5.0Hz), 133.3, 149.8 (J = 5.0 Hz), 152.0 (J = 264.0 Hz);
FABMS m/z 324 (M+H–HCl–2H2O)+, Anal. Calcd forC15H23ClFN3O4S: C, 45.51; H, 5.86; Cl, 8.96; F, 4.80; N, 10.61. Found: C, 45.44; H, 5.65; Cl, 8.87; F, 4.68;N, 10.78.
K-115, an isoquinolinesulfonamide compound, is a highly selective and potent (IC50 = 31 nM) Rho-kinase inhibitor; is in Phase II clinical development in patients with POAG or ocular hypertension.Ripasudil hydrochloride hydrate (Glanatec® ophthalmic solution 0.4 %; hereafter referred to as ripasudil) is a small-molecule, Rho-associated kinase inhibitor developed by Kowa Company, Ltd. for the treatment of glaucoma and ocular hypertension. This compound, which was originally discovered by D. Western Therapeutics Institute, Inc., reduces intraocular pressure (IOP) by directly acting on the trabecular meshwork, thereby increasing conventional outflow through the Schlemm's canal.
Ripasudil hydrochloride hydrate was first approved by Pharmaceuticals and Medical Devices Agency of Japan (PMDA) on Sept 26, 2014. It was developed and marketed as Glanatek® by Kowa Pharmaceuticals.
Ripasudil hydrochloride hydrate is the first drug that can inhibit the rho-associated, coiled-coil containing protein kinase (ROCK). It is indicated for the treatment of glaucoma and ocular hypertension.
Glanatek® is available as solution (0.4%) for ophthalmic use, containing 4 mg of free Ripasudil per millimeter, and the recommended dose is one drop twice daily.
As a result of this mechanism of action, ripasudil may offer additive effects in the treatment of glaucoma and ocular hypertension when used in combination with agents such as prostaglandin analogues (which increase uveoscleral outflow) and β blockers (which reduce aqueous production).
The eye drop product has been approved in Japan for the twice-daily treatment of glaucoma and ocular hypertension, when other therapeutic agents are not effective or cannot be administered. Phase II study is underway for the treatment of diabetic retinopathy.
K-115 is a Rho-kinase inhibitor as ophthalmic solution originally developed by Kowa and D Western Therapeutics Institute (DWTI). The product candidate was approved and launched in Japan for the treatment of glaucoma and ocular hypertension in 2014.
In 2002, the compound was licensed to Kowa Pharmaceutical by D Western Therapeutics Institute (DWTI) in Japan for the treatment of glaucoma. The compound is currently in phase II clinical trials at the company for the treatment of age-related macular degeneration and diabetic retinopathy.
Use of (S)-(-)-1-(4- fluoro-5-isoquinoline-sulfonyl)-2-methyl-1,4-homopiperazine (ripasudil hydrochloride, first disclosed in WO9920620), in the form of eye drops, for the treatment of retinal diseases, particularly diabetic retinopathy or age-related macular degeneration.
Follows on from WO2012105674 by claiming a combination of the same compound. Kowa, under license from D Western Therapeutics Institute, has developed the Rho kinase inhibitor ripasudil hydrochloride hydrate (presumed to be Glanatek) as an eye drop formulation for the treatment of glaucoma and ocular hypertension which was approved in Japan in September 2014..
The company is also developing the agent for the treatment of diabetic retinopathy, for which it is in phase II trial as of October 2014.
Ripasudil (Glanatec) is a drug used for the treatment of glaucoma and ocular hypertension. It is approved for use in Japan as a 0.4% ophthalmic solution.[1]
Paper
A Practical Synthesis of (S)-tert-butyl 3-methyl-1,4-diazepane-1-carboxylate, the key intermediate of Rho-kinase inhibitor K-115
Synthesis (Stuttgart) 2012, 44(20): 3171
Synthesis (Stuttgart) 2012, 44(20): 3171
practical synthesis of (S)-tert-butyl 3-methyl-1,4-diazepane-1-carboxylate has been established for supplying this key intermediate of Rho–kinase inhibitor K-115 in a multikilogram production. The chiral 1,4-diazepane was constructed by intramolecular Fukuyama–Mitsunobu cyclization of a N-nosyl diamino alcohol starting from the commercially available (S)- or (R)-2-aminopropan-1-ol. In the same manner, an enantiomeric pair of a structural isomer were prepared for demonstration of the synthetic utility.
SEE
WO 2006137368 http://www.google.com/patents/WO2006137368A1?cl=en
PATENT
WO 2012026529
The including prevention and treatment cerebral infarction, cerebral hemorrhage, subarachnoid hemorrhage, cerebrovascular disorders such as cerebral edema, the present invention relates to a salt thereof or isoquinoline derivatives useful as therapeutic agents, particularly glaucoma.
(S) - (-) -1 - (4 - fluoro-iso-5 - yl) sulfonyl - 2 - methyl -1,4 - diazepane the following formula (1):
It is a compound represented by the particular it is a crystalline water-soluble, not hygroscopic, because it is excellent in chemical stability, it is useful as a medicament has been known for its hydrochloride dihydrate ( refer to Patent Documents 1 and 2). -5 Isoquinoline of these - the sulfonamide compounds, that prophylactic and therapeutic agents for cerebral infarction, cerebral hemorrhage, subarachnoid hemorrhage, cerebrovascular disorders such as cerebral edema, is useful as a therapeutic agent for preventing and glaucoma in particular is known (1-5 see Patent Document 1).
Conventionally, for example, a method of manufacturing by the method described in Patent Document 1, as shown in the following production process has been reported preparation of said compound (Production Method 1-A).
That is, (S)-1-tert-butoxycarbonyl - 3 - by reacting the presence of triethylamine in methylene chloride-fluoro-isoquinoline (2) - methyl -1,4 - diazepane and 5 (3) - chloro-sulfonyl -4 by adding trifluoroacetic acid in methylene chloride compound (the first step), obtained following (4) to synthesize a compound (4) by deprotection to (second step) the desired compound (1) This is a method of manufacturing.
It is also an important intermediate for preparing the compound (1) (S)-1-tert-butoxycarbonyl - 3 - methyl-1 ,4 - diazepane to (3), for example, in the following manner (; see JP Production Process 1-B) that can be produced is known.
Further, on the other hand, the compound (1) (see Patent Document 1) to be manufactured manufacturing routes such as: Any (Process 2) are known.
WO 1999/20620 pamphlet WO 2006/057397 pamphlet WO 1997/028130 pamphlet JP Patent Publication No. 2006-348028 JP Patent Publication No. 2006-290827
However, it is possible to produce in the laboratory of a small amount scale, but you place the point of view for mass industrial production, environmentally harmful halogenated hydrocarbon solvent in the compound of the above-mentioned process for producing 1-A is ( problem because it is carried out coupling step (3) and 2), giving significant adverse environmental exists.Therefore, solvent of halogenated hydrocarbon other than those listed to the specification of the patent document 1, for example, I tried actually dioxane, tetrahydrofuran and the like, but the present coupling reaction will be some progress indeed, Problems reaction is not completed raw material remained even after prolonged reaction time, yield undesirably stays in at most 30% was found.Furthermore, it is hard to decompose in the environment, elimination is also difficult to dioxane is not preferred irritating to humans, and are known as compounds that potentially harmful brain, kidney and liver .
When we actually produced compound (3) by the above production method 1-B, can be obtained desired compound in good yield merged with reproducibility is difficult has further been found that. That is, in the production path, 1,4 - and is used sodium hydride with dimethyl sulfoxide in forming a diazepane ring, except that I actually doing this step, Tsu than the reproducibility of the desired compound It could not be obtained in high yield Te. Also, that this is due to the synthetic route through the unstable intermediate, that it would be converted into another compound easily found this way. limitations and potential problems of the present production process is exposed since this stability may affect the reproducibility of the reaction.
Meanwhile, an attempt to carry out mass production is actually in the Process 2, it encounters various problems. For example, it is stored as an impurity whenever I repeat step, by-products formed in each stage by tandem production process ranging from step 8 gave more complex impurity profile. Depending, it is necessary to repeat a complicated recrystallization purity obtained as a medicine until the purification, the yield in the laboratory be a good overall yield is significantly reduced in the mass production of actual example be away, it does not have industrial utility of true was found. It can be summarized as follows: Considering from the viewpoint of GMP process control required for pharmaceutical production these problems.
Requires control process and numerous complex ranging 1) to 8 step, 3 2) third step - amino-1 - in the step of reacting a propanol, a difficult to remove positional isomers are mixed, 3) The fourth step water is mixed by the minute liquid extraction operation at the time of return to the free base from oxalate require crystallization purification by oxalate in the removal of contaminants of positional isomers, in 4) fifth step, 5) sixth step The Mitsunobu by reproducibility poor require water control in the Mitsunobu reaction used in the ring closure compounds to (1) compounds in (6), 6) ring closure reaction, departing management of the reagent added or the like is generated, in 7) Seventh Step it takes a complicated purification in impurity removal after the reaction, resulting in a decrease in isolated yield. These are issues that must be solved in order to provide a stable supply of raw material for pharmaceuticals high chemical purity is required.
Thus, gentle salt thereof, or the environment isoquinoline derivative comprising a compound represented by the formula (1), the present invention provides a novel production method having good reproducibility and high purity easily and in high yield I intended.
As a result of intensive studies in view of such circumstances, the present inventors, in the manufacturing process of the final target compound shown by the following expression
(Wherein represents a fluorine, chlorine, bromine or iodine, may, R 3 and 1, R 2 R represents a C 1-4 alkyl group be the same or different from each other, and P, X 1 is a protecting group shows a, 0 to m represents an integer of 3, 0 to n is. represents an integer of 3)
Is a urea-based solvents nitrile solvents, amide solvents, sulfoxide or solvents, the solvent may be preferably used in the coupling step of the compound (III) and (II) are generally very short time With these solvents It has been found that can be converted to the desired product quantitatively. It is possible to carry out the coupling step Volume scale while maintaining a high yield by using these solvents, there is no need to use a halogenated hydrocarbon solvent to give significant adverse environment. In consideration of the process such as removal of the solvent after the reaction was further found that acetonitrile is the best among these solvents. Also, since by using hydrochloric acid with ethyl acetate solvent in step deprotection can be isolated as crystal of hydrochloride desired compound (I), without going through the manipulation of solvent evaporation complicated , it has been found that it is possible to obtain the object compound (I) is a simpler operating procedure. Since there is no need to use a halogenated hydrocarbon solvent in this deprotection step further, there is no possibility of harming the environment.
It has been found that it is possible in mass production of (II), leading to the target compound purity, in high yield with good reproducibility as compared with the conventional method compounds are important intermediates in the coupling step further. That is, was it possible to lead to the intermediate high purity and in high yield by eliminating the production of a harmful halogenated hydrocarbon solvent to the environment in this manner. 1,4 addition - in order to avoid the problems encountered in the reaction using sodium hydride in dimethyl sulfoxide in forming the diazepane ring, in order to allow the cyclization reaction at mild conditions more, as a protecting group By performing the Mitsunobu reaction using Noshiru group instead of the carbobenzyloxy group, in addition to one step shorten the manufacturing process of the whole, without deteriorating the optical purity was successfully obtained the desired compound desired.

SEE
CLIP
Ripasudil hydrochloride hydrate (Glanatec )
Ripasudil hydrochloride hydrate (Glanatec ) was approved in Japan in 2014 for the treatment of glaucoma and ocular hypertension.
219 Originally discovered by D. Western Therapeutics Institute,Inc. and licensed by the Kowa Company, Ltd, ripasudil
functions as a selective Rho-kinase inhibitor and reduces intraocular pressure by stimulation of aqueous humour drainage of the
trabecular meshwork.219–221
Ripasudil hydrochloride hydrate (Glanatec ) was approved in Japan in 2014 for the treatment of glaucoma and ocular hypertension.
219 Originally discovered by D. Western Therapeutics Institute,Inc. and licensed by the Kowa Company, Ltd, ripasudil
functions as a selective Rho-kinase inhibitor and reduces intraocular pressure by stimulation of aqueous humour drainage of the
trabecular meshwork.219–221
While this recent approval allows for use of ripasudil as a twice-daily monotherapy treatment when
other drugs cannot be used or are not effective, clinical trials using ripasudil as a combination therapy with other glaucoma
drugs have shown promising results in the treatment of primary open-angle glaucoma or ocular hypertension.222,223 Currently, the
Kowa Company is also pursuing trials focused on the use of ripasudil for the treatment of diabetic retinopathy and diabetic macular edema.224
other drugs cannot be used or are not effective, clinical trials using ripasudil as a combination therapy with other glaucoma
drugs have shown promising results in the treatment of primary open-angle glaucoma or ocular hypertension.222,223 Currently, the
Kowa Company is also pursuing trials focused on the use of ripasudil for the treatment of diabetic retinopathy and diabetic macular edema.224
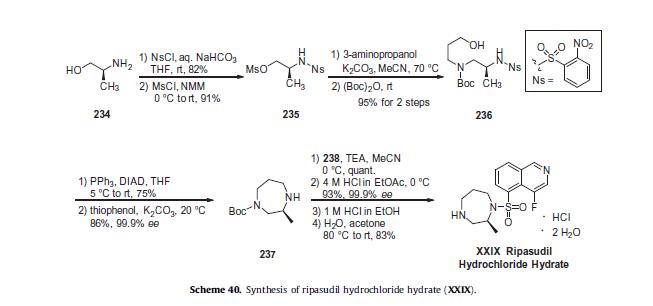
While initial synthetic routes to ripasudil were carried out via a stepwise functionalization of 4-fluoroisoquinoline-5-sulfonylchloride (238),225,226 more recent reports describe an efficient route to ripasudil employing a late stage-coupling of Boc-diazepane
(237) with 4-fluoroisoquinoline-5-sulfonyl chloride (238), enabling synthesis on multi-kilogram scale and isolation of the
drug in high purity (Scheme 40).221,227,228 This optimized route to ripasudil begins with 2-nitrobenzene sulfonyl chloride (NsCl)-
mediated protection of (S)-2-amino-1-propanol (234) in 82% yield.
In this case, use of the NaHCO3/THF/H2O conditions were essential for preventing bis-nosylation.228 Alcohol activation with methanesulfonyl chloride (MsCl) in N-methyl morpholine (NMM) took place smoothly to give the corresponding mesylate 235 in 91%
yield. Direct mesylate displacement with 3-aminopropanol and subsequent amine protection as the carbamate ((Boc)2O) in a
one-pot fashion provided the corresponding Boc-amino propanol product 236 in 95% yield over 2 steps.
(237) with 4-fluoroisoquinoline-5-sulfonyl chloride (238), enabling synthesis on multi-kilogram scale and isolation of the
drug in high purity (Scheme 40).221,227,228 This optimized route to ripasudil begins with 2-nitrobenzene sulfonyl chloride (NsCl)-
mediated protection of (S)-2-amino-1-propanol (234) in 82% yield.
In this case, use of the NaHCO3/THF/H2O conditions were essential for preventing bis-nosylation.228 Alcohol activation with methanesulfonyl chloride (MsCl) in N-methyl morpholine (NMM) took place smoothly to give the corresponding mesylate 235 in 91%
yield. Direct mesylate displacement with 3-aminopropanol and subsequent amine protection as the carbamate ((Boc)2O) in a
one-pot fashion provided the corresponding Boc-amino propanol product 236 in 95% yield over 2 steps.
With the acyclic diazepane precursor 236 in hand, employment of the intramolecular Fukuyama-Mitsunobu N-alkyl cyclization conditions (diisopropylazodicarboxylate (DIAD)/PPh3) allowed generation of the diazepane in 75% yield. Nosyl group cleavage with thiophenol/K2CO3provided the Boc-diazepane 237 in 65% overall yield and 98% purity following a pH-controlled aqueous workup.
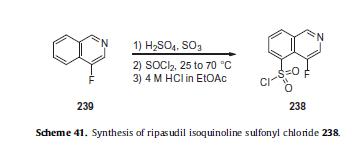
Finally, 4-fluoroisoquinoline- 5-sulfonyl chloride (238)—prepared via subjection of 4- fluoroisoquinoline (239, Scheme 41)229 to sulfur trioxide and sulfuric acid followed by treatment with thionyl chloride and finally 4 N HCl in ethyl acetate—was involved in a 1-pot, two-step procedure in which this sulfonyl chloride was coupled with diazepane 237 (TEA/MeCN) to access the ripasudil framework in quantitative yield.
Synthesis of the final drug target by deprotection with 4 MHCl in ethyl acetate followed by neutralization with aqueoussodium hydroxide provided the free base of ripasudil in 93% yield and 99.8% purity. Conversion to the more stable hydrochloride dihydrate form could be performed by treatment of the free base with 1 M HCl/EtOH and subsequent heating of the hydrochloride in H2O/acetone to provide ripasudil hydrochloride dihydrate XXIX in 83% yield.230,231
219. Garnock, J. P. K. Drugs 2014, 74, 2211.
220. Isobe, T.; Mizuno, K.; Kaneko, Y.; Ohta, M.; Koide, T.; Tanabe, S. Curr. Eye Res.2014, 39, 813.
221. Sumi, K.; Inoue, Y.; Nishio, M.; Naito, Y.; Hosoya, T.; Suzuki, M.; Hidaka, H.
Bioorg. Med. Chem. Lett. 2014, 24, 831.
222. Mizuno, K. WO Patent 2,012,105,674, 2012.
223. Mizuno, K.; Matsumoto, J. WO Patent 2,007,007,737, 2007.
224. http://clinicaltrials.jp/user/cteDetail.jsp.
225. Gomi, N.; Ohgiya, T.; Shibuya, K. WO Patent 2,012,026,529, 2012.
226. Hidaka, H.; Nishio, M.; Sumi, K. US Patent 20,080,064,681, 2008.
227. Gomi, N.; Kouketsu, A.; Ohgiya, T.; Shibuya, K. Synthesis 2012, 44, 3171.
228. Gomi, N.; Ohgiya, T.; Shibuya, K.; Katsuyama, J.; Masumoto, M.; Sakai, H.Heterocycles 2011, 83, 1771.
229. Sakai, H.; Masunoto, M.; Katsuyama, J.; Onogi, K. WO Patent 2006090783A1,2006.
230. Hidaka, H.; Matsuura, A. WO Patent 1999020620A1, 1999.
231. Ohshima, T.; Hidaka, H.; Shiratsuchi, M.; Onogi, K.; Oda, T. US Patent7858615B2, 2008.
220. Isobe, T.; Mizuno, K.; Kaneko, Y.; Ohta, M.; Koide, T.; Tanabe, S. Curr. Eye Res.2014, 39, 813.
221. Sumi, K.; Inoue, Y.; Nishio, M.; Naito, Y.; Hosoya, T.; Suzuki, M.; Hidaka, H.
Bioorg. Med. Chem. Lett. 2014, 24, 831.
222. Mizuno, K. WO Patent 2,012,105,674, 2012.
223. Mizuno, K.; Matsumoto, J. WO Patent 2,007,007,737, 2007.
224. http://clinicaltrials.jp/user/cteDetail.jsp.
225. Gomi, N.; Ohgiya, T.; Shibuya, K. WO Patent 2,012,026,529, 2012.
226. Hidaka, H.; Nishio, M.; Sumi, K. US Patent 20,080,064,681, 2008.
227. Gomi, N.; Kouketsu, A.; Ohgiya, T.; Shibuya, K. Synthesis 2012, 44, 3171.
228. Gomi, N.; Ohgiya, T.; Shibuya, K.; Katsuyama, J.; Masumoto, M.; Sakai, H.Heterocycles 2011, 83, 1771.
229. Sakai, H.; Masunoto, M.; Katsuyama, J.; Onogi, K. WO Patent 2006090783A1,2006.
230. Hidaka, H.; Matsuura, A. WO Patent 1999020620A1, 1999.
231. Ohshima, T.; Hidaka, H.; Shiratsuchi, M.; Onogi, K.; Oda, T. US Patent7858615B2, 2008.
H-NMR spectral analysis
![4-fluoro-5-[[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl]isoquinoline NMR spectra analysis, Chemical CAS NO. 223645-67-8 NMR spectral analysis, 4-fluoro-5-[[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl]isoquinoline H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-28/002/466/2466109_1h.png) CAS NO. 223645-67-8, 4-fluoro-5-[[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl]isoquinoline H-NMR spectral analysis |
C-NMR spectral analysis
![4-fluoro-5-[[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl]isoquinoline NMR spectra analysis, Chemical CAS NO. 223645-67-8 NMR spectral analysis, 4-fluoro-5-[[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl]isoquinoline C-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-11-28/002/466/2466109_13c.png) CAS NO. 223645-67-8, 4-fluoro-5-[[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl]isoquinoline C-NMR spectral analysis |
·
| WO1997028130A1 | Jan 31, 1997 | Aug 7, 1997 | Hiroyoshi Hidaka | Isoquinoline derivatives and drugs |
| WO1999020620A1 | Oct 22, 1998 | Apr 29, 1999 | Hiroyoshi Hidaka | Isoquinoline derivative and drug |
| WO2006057397A1 | Nov 29, 2005 | Jun 1, 2006 | Hiroyoshi Hidaka | (s)-(-)-1-(4-fluoroisoquinolin-5-yl)sulfonyl-2-methyl-1,4homopiperazine hydrochloride dihydrate |
| JP2006290827A | Title not available | |||
| JP2006348028A | Title not available | |||
| JPH11171885A * | Title not available | |||
| JPS61227581A * | Title not available |
References
- Garnock-Jones, K. P. (2014). "Ripasudil: First global approval". Drugs 74 (18): 2211–5. doi:10.1007/s40265-014-0333-2.PMID 25414122.
- Tanihara, H; Inoue, T; Yamamoto, T; Kuwayama, Y; Abe, H; Suganami, H; Araie, M; the K-115 Clinical Study Group (2014). "Intra-ocular pressure-lowering effects of a Rho kinase inhibitor, ripasudil (K-115), over 24 hours in primary open-angle glaucoma and ocular hypertension: A randomized, open-label, crossover study". Acta Ophthalmologica: n/a. doi:10.1111/aos.12599. PMID 25487877.
 | |
| Systematic (IUPAC) name | |
|---|---|
| 4-Fluoro-5-{[(2S)-2-methyl-1,4-diazepan-1-yl]sulfonyl}isoquinoline | |
| Clinical data | |
| Trade names | Glanatec |
| Identifiers | |
| PubChem | CID 9863672 |
| ChemSpider | 8039366 |
| Synonyms | K-115 |
| Chemical data | |
| Formula | C15H18FN3O2S |
| Molar mass | 323.39 g/mol |
///////////////// , Ripasudil hydrochloride hydrate, Ripasudil, 223645-67-8, 塩酸塩水和物 , リパスジル
O=S(=O)(c2c1c(F)cncc1ccc2)N3[C@H](CNCCC3)C