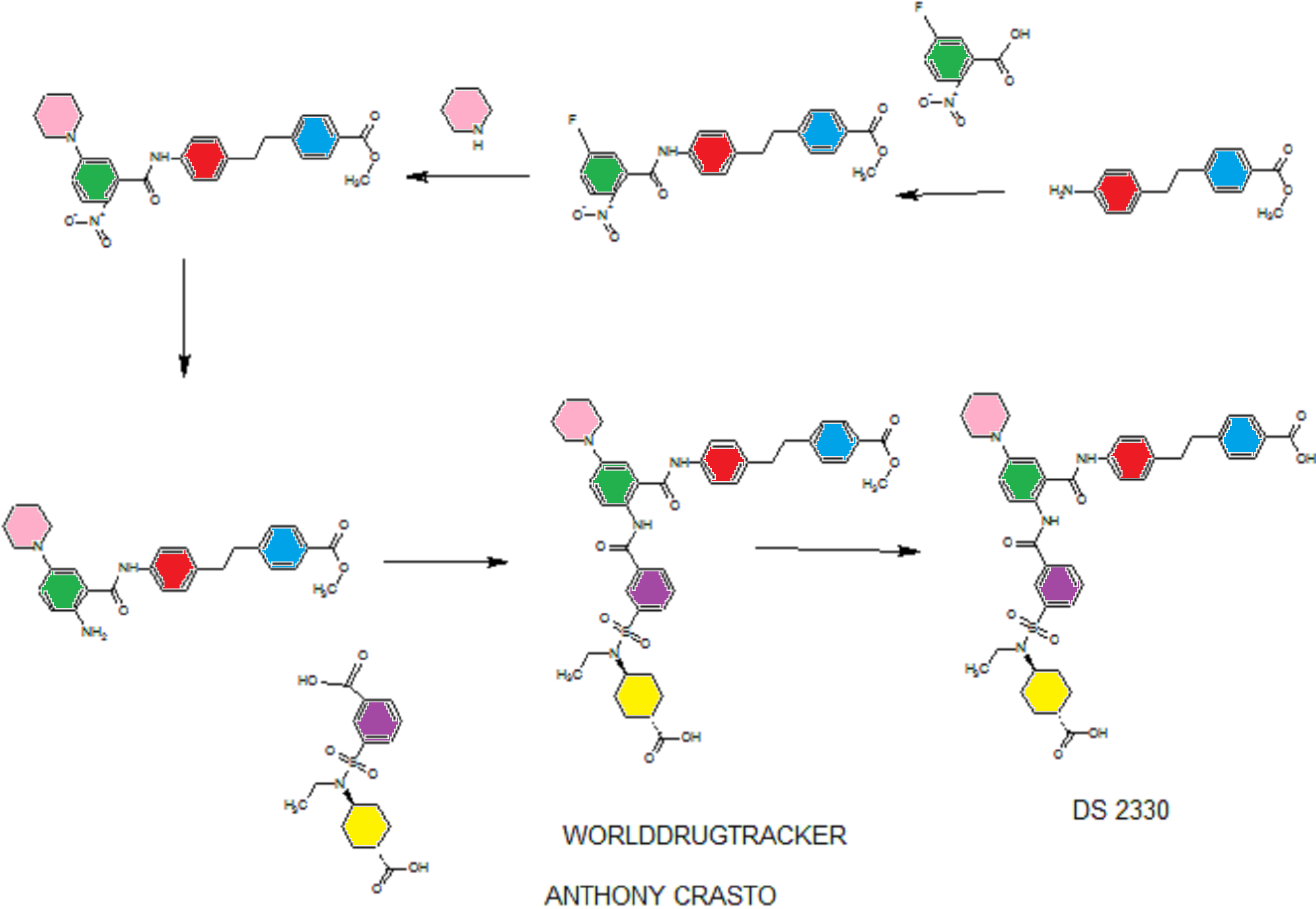
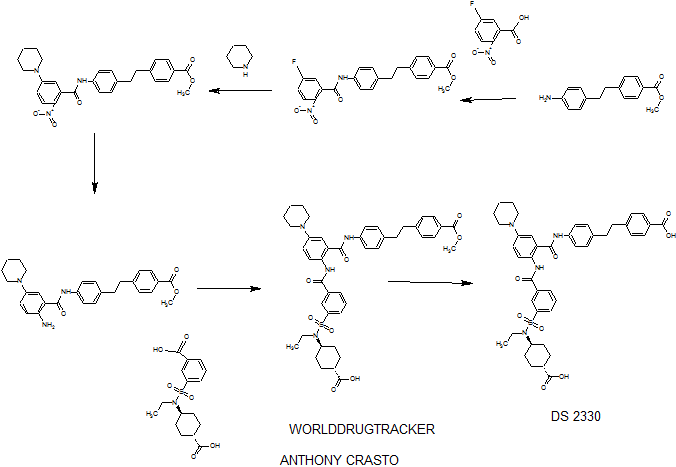
DS 2330
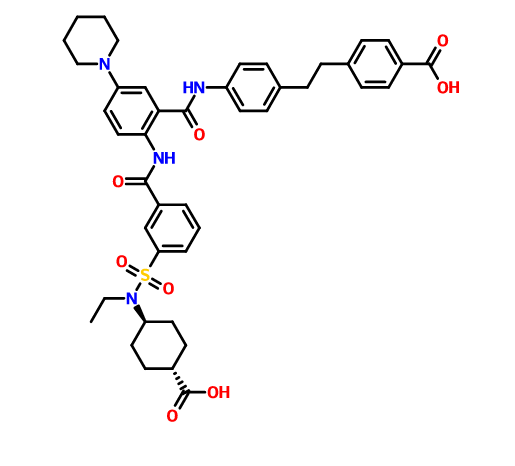
4-[2-(4-{[2-({3-[(trans-4-carboxy-cyclohexyl)(ethyl)sulfocarbamoyl]benzoyl}amino)-5-(piperidin-1-yl)benzoyl]amino}phenyl)ethyl]benzoic acid,
4- [2- (4 - {[2 - ({3 - [(trans-4-carboxy-cyclohexyl) (ethyl) sulfur carbamoyl] benzoyl} amino) -5- (piperidin-1-yl) benzoyl] amino} phenyl) ethyl] benzoate
CAS 1634680-81-1
C43 H48 N4 O8 S, 780.9
Benzoic acid, 4-[2-[4-[[2-[[3-[[(trans-4-carboxycyclohexyl)ethylamino]sulfonyl]benzoyl]amino]-5-(1-piperidinyl)benzoyl]amino]phenyl]ethyl]-
CIS CAS 1634681-85-8
DISODIUM SALT 1634681-00-7
- OriginatorDaiichi Sankyo Inc
- ClassHyperphosphataemia therapies
useful for treating hyperphosphatemia, DS-2330, a phosphorous lowering agent, being developed by Daiichi Sankyo, for treating hyperphosphatemia in chronic kidney disease. In April 2016, DS-2330 was reported to be in phase 1 clinical development.
- Phase IHyperphosphataemia
- 31 Oct 2015Phase-I clinical trials in Hyperphosphataemia in USA (unspecified route)

SEE WO2015108038,
PATENT
WO2014175317
PATENT
he problem is to provide a pharmaceutical for the prevention or treatment of hyperphosphatemia. The solution is a salt of a compound including formula (I), or a crystal of a hydrate thereof.
(Example 1)
disodium 4- [2- (4 - {[2 - ({3 - [(trans-4-carboxy-cyclohexyl) (ethyl) sulfur carbamoyl] benzoyl} amino) -5- (piperidin-1-yl ) benzoyl] amino} phenyl) ethyl] benzoic acid trihydrate
Disodium 4- [2- (4 - { [2 - ({3 - [(trans-4-carboxylatocyclohexyl) (ethyl) sulfamoyl] benzoyl} amino) - 5- (piperidin-1-yl) benzoyl] amino} phenyl) ethyl] benzoate trihydrate
of α crystal
disodium 4- [2- (4 - {[2 - ({3 - [(trans-4-carboxy-cyclohexyl) (ethyl) sulfur carbamoyl] benzoyl} amino) -5- (piperidin-1-yl ) benzoyl] amino} phenyl) ethyl] benzoic acid trihydrate
Disodium 4- [2- (4 - { [2 - ({3 - [(trans-4-carboxylatocyclohexyl) (ethyl) sulfamoyl] benzoyl} amino) - 5- (piperidin-1-yl) benzoyl] amino} phenyl) ethyl] benzoate trihydrate
of α crystal
[Formula 7] crystal of disodium salt trihydrate of (α crystal)

(1)
4- [2- (4 - {[2 - ({3 - [(trans-4-carboxy-cyclohexyl) (ethyl) sulfur carbamoyl] benzoyl} amino) -5- (piperidin-1-yl) benzoyl] amino} phenyl) ethyl] 1 mol / L NaOH aqueous solution to benzoic acid (1.2 g) (3.1 mL) was added and dissolved completely. After stirring at room temperature for 1 day was added acetonitrile (60 mL), at 40 ° C.
and stirred for further 1 day. The precipitated solid was collected by filtration, and 3 hours drying under reduced pressure at room temperature to give the title compound 1.1 g (85%).
4- [2- (4 - {[2 - ({3 - [(trans-4-carboxy-cyclohexyl) (ethyl) sulfur carbamoyl] benzoyl} amino) -5- (piperidin-1-yl) benzoyl] amino} phenyl) ethyl] 1 mol / L NaOH aqueous solution to benzoic acid (1.2 g) (3.1 mL) was added and dissolved completely. After stirring at room temperature for 1 day was added acetonitrile (60 mL), at 40 ° C.
and stirred for further 1 day. The precipitated solid was collected by filtration, and 3 hours drying under reduced pressure at room temperature to give the title compound 1.1 g (85%).
(2)
4- [2- (4 - {[2 - ({3 - [(trans-4-carboxy-cyclohexyl) (ethyl) sulfur carbamoyl] benzoyl} amino) -5- (piperidin-1-yl) benzoyl] amino} phenyl) ethyl] benzoate (40.0 g)
in water (46.4 mL), 1-PrOH (72 mL), 4 mol / L NaOH aqueous solution (25.54 mL) was added, then filtered after stirring insolubles at room temperature, water / 1-PrOH: was washed with (3 7, 80 mL). The filtrate was heated up to 40 ℃, 1-PrOH the (160 mL) was added, and further seed crystal (α crystals, 0.2g) was added. Then the temperature was raised to 50 ℃, 1-PrOH (96 ml) was added, and the mixture was stirred overnight.Thereafter, 1-PrOH (480 ml) was added and after overnight stirring, was collected by filtration the precipitated solid was cooled to room temperature.Thereafter, and vacuum dried overnight at 40 ° C., to give the title compound 39.4 g (96%).
in water (46.4 mL), 1-PrOH (72 mL), 4 mol / L NaOH aqueous solution (25.54 mL) was added, then filtered after stirring insolubles at room temperature, water / 1-PrOH: was washed with (3 7, 80 mL). The filtrate was heated up to 40 ℃, 1-PrOH the (160 mL) was added, and further seed crystal (α crystals, 0.2g) was added. Then the temperature was raised to 50 ℃, 1-PrOH (96 ml) was added, and the mixture was stirred overnight.Thereafter, 1-PrOH (480 ml) was added and after overnight stirring, was collected by filtration the precipitated solid was cooled to room temperature.Thereafter, and vacuum dried overnight at 40 ° C., to give the title compound 39.4 g (96%).
REFERENCES
////////////DS 2330, DS-2330, DAIICHI SANKYO, phase 1
O=C(O)[C@@H]1CC[C@H](CC1)N(CC)S(=O)(=O)c2cccc(c2)C(=O)Nc5ccc(cc5C(=O)Nc4ccc(CCc3ccc(cc3)C(=O)O)cc4)N6CCCCC6
OR
O=C(O)[C@@H]1CC[C@H](CC1)N(CC)S(=O)(=O)c2cccc(c2)C(=O)Nc5ccc(cc5C(=O)Nc4ccc(CCc3ccc(cc3)C(=O)O)cc4)N6CCCCC6