S-2474
(E)-(5)-(3,5-Di-tert-butyl-4-hydroxybenzylidene)-2-ethyl-1,2-isothiazolidine-1,1-dioxide
Shionogi Research Laboratories
cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LO)
S-2474,158089-95-3, 158089-96-4 ((Z)-isomer),C20-H31-N-O3-S,
E)-5-(3,5-Di-tert-butyl-4-hydroxybenzylidene)-2-ethylisothiazolidine 1,1-dioxide
- Phenol, 2,6-bis(1,1-dimethylethyl)-4-[(2-ethyl-5-isothiazolidinylidene)methyl]-, S,S-dioxide, (E)-
- 2,6-Bis(1,1-dimethylethyl)-4-[(E)-(2-ethyl-1,1-dioxido-5-isothiazolidinylidene)methyl]phenol
- Phenol, 2,6-bis(1,1-dimethylethyl)-4-[(2-ethyl-1,1-dioxido-5-isothiazolidinylidene)methyl]-, (E)-
(E)-(5)-(3,5-Di-tert-butyl-4-hydroxybenzylidene)-2-ethyl-1,2-isothiazolidine-1,1-dioxide (S-2474, ), which was discovered at Shionogi Research Laboratories, shows potent inhibitory effects on both cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LO) and is anticipated to be promising as an antiarthritic drug

synthesis of novel γ-sultam derivatives containing the di-tert-butylphenol antioxidant moiety. Several compounds with lower alkyl groups at the 2-position of the γ-sultam skeleton showed potent inhibitory activities against PGE2 production via the COX pathway and LTB4 production via the 5-LO pathway, as well as production of IL-1 in in vitro assays. Extensive pharmacological characterizations revealed that 2-ethyl-γ-sultam derivative 10b displays multiple inhibition of COX, 5-LO, and IL-1 production similar to tenidap and also good selective COX-2 inhibition like NS-398 and celecoxib. It exerted excellent antiinflammatory activity without any ulcerogenic effects and was designated as S-2474 an agent having both NSAID and cytokine modulating properties. S-2474 is now being developed as a promising alternative antiarthritic drug candidate
SYNTHESIS
17th Symp Med Chem (Nov 19 1997 , Tsukuba), EP 0595546; JP 1994211819; US 5418230
The intermediate gamma-sultam (III) was prepared by condensation of 3-chloropropylsulfonyl chloride (I) with ethylamine, followed by cyclization of the resulting chloro sulfonamide (II) under basic conditions. Condensation of 3,5-di- tert-butyl-4- (methoxymethoxy) benzaldehyde (IV) with sultam (III) in the presence of LDA produced the aldol addition compound (V). Then, acid-promoted dehydration and simultaneous methoxymethyl group deprotection gave rise to a mixture of the desired E-benzylidene sultam and the corresponding Z-isomer (VII), which were separated by column chromatography.

PAPER
Novel Antiarthritic Agents with 1,2-Isothiazolidine-1,1-dioxide (γ-Sultam) Skeleton: Cytokine Suppressive Dual Inhibitors of Cyclooxygenase-2 and 5-Lipoxygenase
Shionogi Research Laboratories, Shionogi & Co., Ltd., Fukushima-ku, Osaka 553-0002, Japan, and Institute of Medical Science, St. Marianna University School of Medicine, Miyamae-ku, Kawasaki 216-8512, Japan
J. Med. Chem., 2000, 43 (10), pp 2040–2048
DOI: 10.1021/jm9906015

Various 1,2-isothiazolidine-1,1-dioxide (γ-sultam) derivatives containing an antioxidant moiety, 2,6-di-tert-butylphenol substituent, were prepared. Some compounds, which have a lower alkyl group at the 2-position of the γ-sultam skeleton, showed potent inhibitory effects on both cyclooxygenase (COX)-2 and 5-lipoxygenase (5-LO), as well as production of interleukin (IL)-1 in in vitro assays. They also proved to be effective in several animal arthritic models without any ulcerogenic activities. Among these compounds, (E)-(5)-(3,5-di-tert-butyl-4-hydroxybenzylidene)-2-ethyl-1,2-isothiazolidine-1,1-dioxide (S-2474) was selected as an antiarthritic drug candidate and is now under clinical trials. The structure−activity relationships (SAR) examined and some pharmacological evaluations are described.
PAPER
Highly E-Selective and Effective Synthesis of Antiarthritic Drug Candidate S-2474 Using Quinone Methide Derivatives
Shionogi Research Laboratories, Shionogi & Company, Ltd., Fukushima-ku, Osaka 553-0002, Japan
J. Org. Chem., 2002, 67 (1), pp 125–128
DOI: 10.1021/jo0106795

We have developed an efficient and E-selective synthesis of an antiarthritic drug candidate (E)-(5)-(3,5-di-tert-butyl-4-hydroxybenzylidene)-2-ethyl-1,2-isothiazolidine-1,1-dioxide (S-2474), in which α-methoxy-p-quinone methide is used as a key intermediate. α-Methoxy-p-quinone methide was revealed to be an equiv. to a p-hydroxy protected benzaldehyde. It reacts smoothly with α-sulfonyl carbanion to give 1,6-addn. intermediates, which can be further processed to provide S-2474 directly in the presence of a base. This procedure gives S-2474 as an almost single isomer on the benzylidene double bond in excellent yield and thus is a very practical method adaptable to large-scale synthesis. The detailed mechanistic aspects are studied and discussed.
An improved synthesis has been reported. Acid -catalyzed ketalization of aldehyde (VIII) with trimethyl orthoformate provided the dimethyl acetal (IX) which, upon thermal decomposition in refluxing xylene, gave rise to the alpha-methoxy methylenequinone derivative (X ). This was then condensed with the lithio derivative of sultam (III) to form selectively the desired E-adduct. in an analogous procedure, aldehyde (VIII) was converted to the chloromethylene compound (XI) with methanesulfonyl chloride and triethylamine in refluxing CH2Cl2 . Condensation of (XI) with the lithiated sultam (III) furnished the desired E-benzylidene sultam.

PAPER
Development of One-Pot Synthesis of New Antiarthritic Drug Candidate S-2474 with High E-Selectivity
Chemical Development Department, CMC Development Laboratories, Shionogi & Co., Ltd., 1-3, Kuise Terajima 2-chome, Amagasaki, Hyogo 660-0813, Japan, and Shionogi Research Laboratories, Shionogi & Co., Ltd., 12-4, Sagisu 5-chome, Fukushima-ku, Osaka 553-0002, Japan
Org. Process Res. Dev., 2008, 12 (3), pp 442–446
DOI: 10.1021/op800008w
* To whom correspondence should be addressed. Telephone: +81-6-6401-8198 . Fax: +81-6-6401-1371. E-mail:takemasa.hida@shionogi.co.jp., †
Chemical Development Department, CMC Development Laboratories.
, ‡Shionogi Research Laboratories.
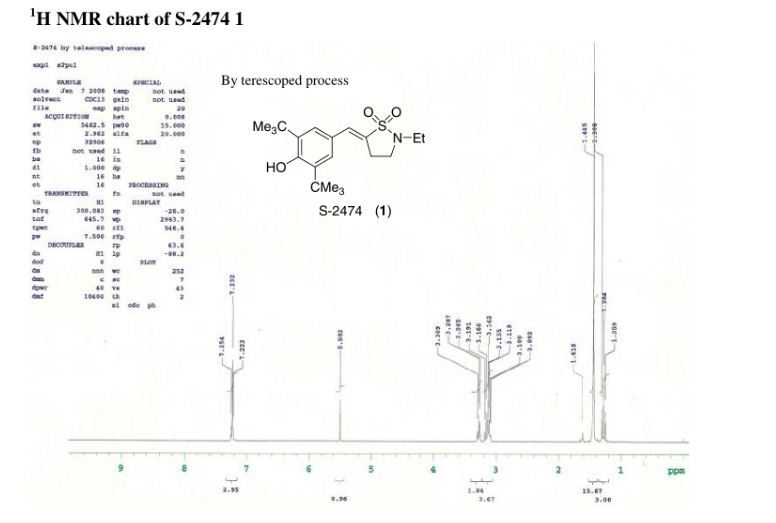
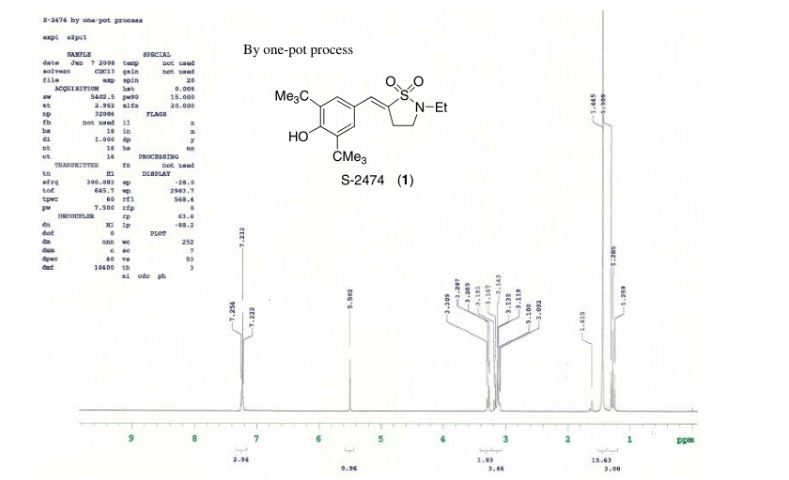
A one-pot synthesis of S-2474 was developed to overcome the problems of a large number of steps, low stereoselectivity, low yield, a large amount of waste, and severe reaction conditions. Aldol-type condensation of 3,5-di-tert-butyl-4-hydroxybenzaldehyde and N-ethyl-γ-sultam was carried out with LDA and then quenched with water. Dehydration proceeded under basic conditions, providing S-2474 directly as a single isomer on the benzylidene double bond. The reaction mechanism appears to involve a quinone methide intermediate. Environmental assessment of the development of this compound is also discussed in this paper.
///////New, Antiarthritic , Drug Candidate, S-2474, Shionogi Research Laboratories, cyclooxygenase-2, (COX-2), 5-lipoxygenase , (5-LO), PHASE 2, 158089-95-3, 158089-96-4, S2474, S 2474
CCN2CC\C(=C/c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)S2(=O)=O