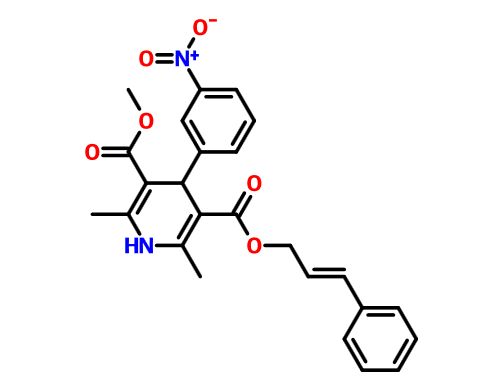

Pranidipine , OPC-13340, FRC 8411
Acalas®
NDA Filing in Japan
A calcium channel blocker potentially for the treatment of angina pectoris and hypertension.
CAS No. 99522-79-9
- Molecular FormulaC25H24N2O6
- Average mass448.468
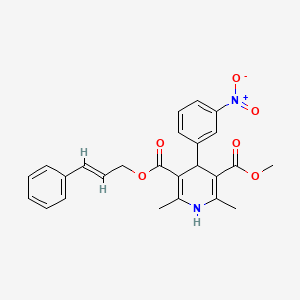
methyl (2E)-3-phenylprop-2-en-1-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
Methyl-(2E)-3-phenyl-2-propen-1-yl-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydro-3,5-pyridindicarboxylat (E)-Cinnamyl methyl (±)-1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
Methyl cinnamyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydro-3,5-pyridinedicarboxylate
trans-Cinnamyl methyl 4-(3-nitrophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, methyl (2E)-3-phenyl-2-propen-1-yl ester
Pranidipine is a calcium channel blocker. It is a long acting calcium channel antagonist of the dihydropyridine group.[1]
PATENT
EP 0173126 http://www.google.com/patents/EP0173126A1?cl=en
PAPER
Der Pharmacia Sinica, 2014, 5(1):11-17
pelagiaresearchlibrary.com/der-pharmacia-sinica/vol5-iss1/DPS-2014-5-1-11-17.pdf
CLICK ON IMAGE FOR CLEAR VIEW
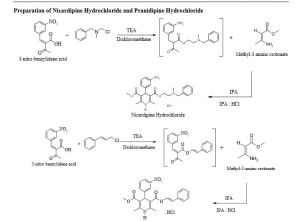
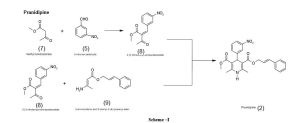
Preparation of Pranidipine Hydrochloride(2):
To a suspension of (Z)-2-(3-nitrobenzylidene)-3-oxobutanoic acid(3) (1.2 kg, 5.10 mol) in dichloromethane (6 L)
was added triethylamine(0.77 kg, 7.65 mol) and cinnamyl chloride (0.85 kg, 5.61 mol). The reaction mixture was
heated to 45°C and maintained for 2 hrs. The suspension was cooled to 25 to 30°C and washed with 2.4 Lof DM
water. DCM layer was separated and concentrated under vacuum below 40°C. The concentrated mass was dissolved
in 7.2 L isopropyl alcohol and methyl-3-amino crotonate (0.52 kg, 4.5mol) was added to it. Temperatureof reaction
mixture was slowly raised to 70°C and maintained for 8 hours. Reaction mass was concentrated under vacuum
below 40°C.To the crude residue, ethyl acetate-HCl(0.28 kg, 7.6 mol) was added and the reaction mixture was
stirred for 24 hours at 25°C-30°C. Reaction mixturewas filtered and the solid residue was dried under
vacuum toafford 1.6 kg of Pranidipine hydrochloride (2)in 85% yield with 98 % purity.
1H-NMR(DMSO):
δ2.29 (s, 3H),2.32 (s, 3H), 3.55 (s, 3H), 4.60-4.74 (m, 2H), 5.04(s, 1H), 6.26-6.33 (m, 1H), 6.50 (d, 1H), 7.24-7.3
8 (m, 5H), 7.53(t, 1H), 7.63 (d,1H), 7.98-8.01 (m, 1H), 9.08 (brs, 1H)
Preparation of (Z)-2-(3-nitrobenzylidene)-3-oxobutanoic acid(3):
To a suspension of (Z)-t-butyl 2-(3-nitrobenzylidene)-3-oxobutanoate(10) (1.5 kg, 5.14 mol) in dichloromethane
(7.5 L) was added trifluoroacetic acid (1.76 kg, 15.44 mol) and reaction mass was stirred at 25°C to 30°C for 24 hrs.
The reaction mass was concentrated under vacuum below 40°C and stripped with toluene. The concentratedmass
was dissolved in 4.5 L toluene and the solution wasstirred for 8 hours at 25°C to 30°C. Reaction mixture was
filtered and solid washed with toluene and dried at35°C to 40°C to give 1.152 kg of (Z)-2-(3-nitrobenzylidene)-3-
oxobutanoic acid(3) in 96 % yield. M.P: 120°C; Mol.Wt: 235.20; Mol.Formula: C11H9NO5;1H-NMR(DMSO):
δ2.46 (s, 3H), 7.76-7.83 (m, 2H), 8.02 (d, 1H), 8.28-8.31 (dd, 1H), 8.51 (s, 1H), 13.63 (brs, 1H).Anal.Calcd for
C11H10NO5 : C, 55.93; H, 4.27; N, 5.93. Found: C, 56.19;H, 4.09; N, 6.27
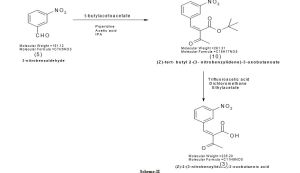
Preparation of (Z)-tertiary- butyl 2-(3-nitrobenzylidene)-3-oxobutanoate(10):
To a suspension of 3-nitrobenzaldehyde(5) (1 kg, 6.61 mol) in isopropyl alcohol (6 L) was addedt-butylacetoacetate (1.14 kg, 7.27 mol),piperidine (0.12 kg, 1.32 mol) and acetic acid (0.79 kg, 1.32 mol). The reactionmass was stirred at 25°C to 30°C for 6 hrs. The suspension was cooled to -5 to 0°C, filtered, residuewashed withisopropyl alcohol and dried at 35°C to 40°C to give
1.750 kg of (Z)-t-butyl 2-(3-nitrobenzylidene)-3-oxobutanoate(10)in 91% yield; M.P: 80°C; Mol. Wt: 291.31; Mol.Formula: C15H17NO5
1H-NMR(CDCl3):
δ1.55(s, 9H), 2.44 (s, 3H), 7.50 (s, 1H),7.59 (t, 1H),7.80 (d, 1H), 8.24- 8.27 (dd,J=1H),δ8.41 (t, 1H).
CLICK ON IMAGE FOR CLEAR VIEW
Patent |
Submitted | Granted |
|---|---|---|
| Process for the preparation of 1,4 - dihydropyridines and novel 1,4-dihydropyridines useful as therapeutic agents [US2003230478] | 2003-12-18 | |
| Advanced Formulations and Therapies for Treating Hard-to-Heal Wounds [US2014357645] | 2014-08-19 | 2014-12-04 |
| METHODS OF TREATING CARDIOVASCULAR AND METABOLIC DISEASES [US2014322199] | 2012-08-06 | 2014-10-30 |
| Protein Carrier-Linked Prodrugs [US2014323402] | 2012-08-10 | 2014-10-30 |
| sGC STIMULATORS [US2014323448] | 2014-04-29 | 2014-10-30 |
| TREATMENT OF ARTERIAL WALL BY COMBINATION OF RAAS INHIBITOR AND HMG-CoA REDUCTASE INHIBITOR [US2014323536] | 2012-12-07 | 2014-10-30 |
| Agonists of Guanylate Cyclase Useful For the Treatment of Gastrointestinal Disorders, Inflammation, Cancer and Other Disorders [US2014329738] | 2014-03-28 | 2014-11-06 |
| METHODS, COMPOSITIONS, AND KITS FOR THE TREATMENT OF CANCER [US2014335050] | 2012-05-25 | 2014-11-13 |
| ROR GAMMA MODULATORS [US2014343023] | 2012-09-18 | 2014-11-20 |
| High-Loading Water-Soluable Carrier-Linked Prodrugs [US2014296257] | 2012-08-10 | 2014-10-02 |
| Publication Number | Publication Date | IPCR Assignee/Applicant | Structure hits | Tools | |
|---|---|---|---|---|---|
1.
US-20150342954-A1 |
2015-12-03 | ||||
2.
EP-2558474-B1 |
2015-11-25 |
EN
|
|||
3.
US-20150307580-A1 |
2015-10-29 | ||||
4.
US-20150305974-A1 |
2015-10-29 | ||||
5.
WO-2015164658-A1 |
2015-10-29 |
EN
|
|||
6.
EP-2527360-B1 |
2015-10-28 |
EN
|
|||
7.
WO-2015157471-A1 |
2015-10-15 |
EN
|
|||
8.
US-20150284411-A1 |
2015-10-08 | ||||
9.
US-20150283202-A1 |
2015-10-08 | ||||
10.
US-9150512-B2 |
2015-10-06 |
References
 |
|
| Names | |
|---|---|
| IUPAC name
methyl (2E)-phenylprop-2-en-1-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
|
|
| Other names
2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid O5-methyl O3-[(E)-3-phenylprop-2-enyl] ester
|
|
| Identifiers | |
| 99522-79-9 |
|
| ChEMBL | ChEMBL1096842 |
| ChemSpider | 4940726 |
| Jmol interactive 3D | Image |
| MeSH | C048161 |
| PubChem | 6436048 |
| UNII | 9DES9QVH58 |
| Properties | |
| C25H24N2O6 | |
| Molar mass | 448.46786 |
SEE.........http://newdrugapprovals.org/2015/12/11/pranidipine/
////////// CC1=C(C(C(=C(N1)C)C(=O)OCC=CC2=CC=CC=C2)C3=CC(=CC=C3)[N+](=O)[O-])C(=O)OC
see dipine series...........http://organicsynthesisinternational.blogspot.in/p/dipine-series.html
 Nilvadipine - Wikipedia, the free encyclopedia
Nilvadipine - Wikipedia, the free encyclopedia