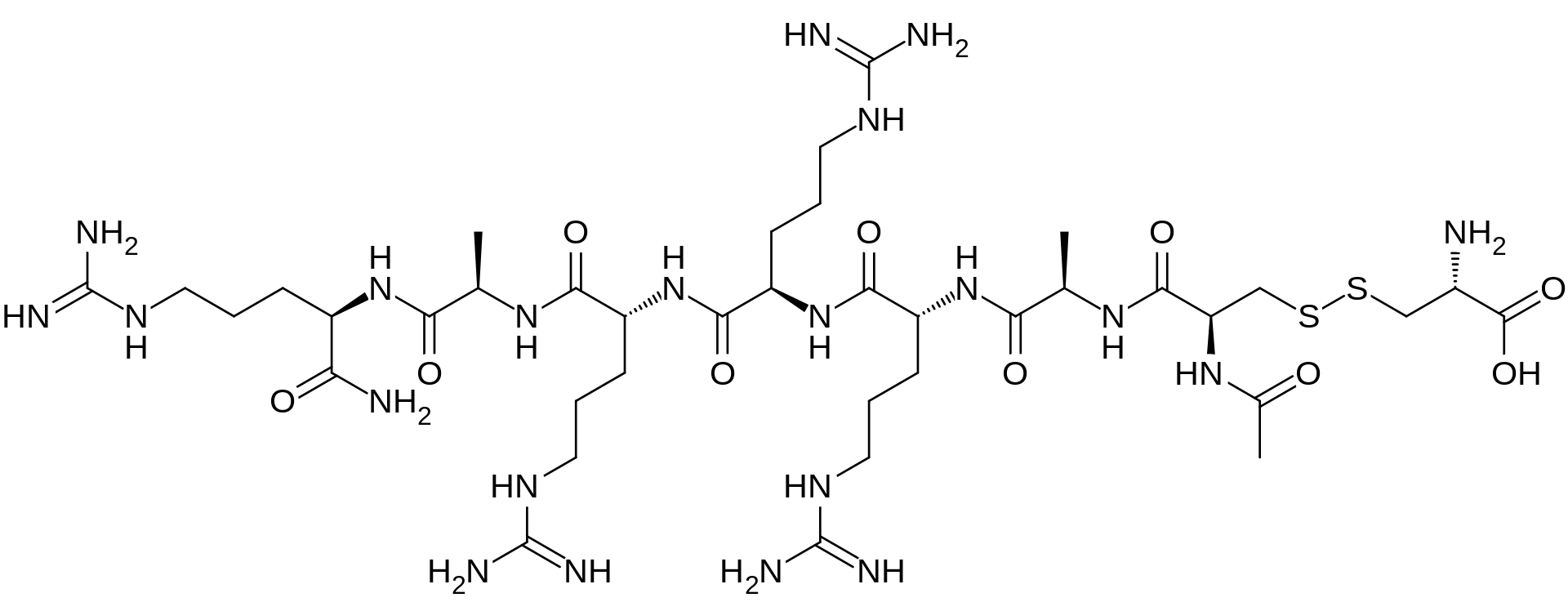
Etelcalcetide, AMG 416
AMG-416; Etelcalcetide hydrochloride; KAI-4169; KAI-4169-HCl; ONO-5163; Telcalcetide; Velcalcetide; Velcalcetide hydrochloride
D-Argininamide, N-acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-, disulfide with L-cysteine,
N-Acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-D-argininamide disulfide with L-cysteine
Secondary hyperparathyroidism
- Originator KAI Pharmaceuticals...Kai Pharmaceuticals, Inc.
- Developer Amgen; KAI Pharmaceuticals; Ono Pharmaceutical
- ClassDisulfides; Peptides
- Mechanism of ActionCalcium-sensing receptor agonists
New Parathyroid Disease Drug Seeks FDA Approval
Amgen is seeking FDA approval for etelcalcetide (AMG 461), the first calcimimetic agent administered intravenously after dialysis to treat secondary hyperparathyroidism (SHPT) in patients with chronic kidney disease (CKD).
SHPT is a common and serious condition that is often progressive among CKD patients. It usually manifests as high amounts of parathyroid hormone (PTH) associated with abnormal calcium and phosphorus levels in the body.
- See more at: http://www.pharmacytimes.com/product-news/new-parathyroid-disease-drug-seeks-fda-approval
- See more at: http://www.pharmacytimes.com/product-news/new-parathyroid-disease-drug-seeks-fda-approval
Etelcalcetide is a D-amino peptide calcimimetic undergoing clinical evaluation for the treatment of secondary hyperparathyroidismfor patients with chronic kidney disease (CKD) on hemodialysis. Etelcalcetide is administered intravenously at the end of each dialysis session.[1][2] It exerts a pharmacological effect by binding to and activating the calcium-sensing receptor (CaSR) in theparathyroid gland, resulting in parathyroid hormone (PTH) reduction and suppression.[1] Elevated PTH is often observe in patients with CKD.[3]
On August 25, 2015 Amgen Inc. announced its submission of a New Drug Application to the Food and Drug Administration for etelcalcetide.[1]
| CAS Registry Number | 1262780-97-1 |
|---|---|
| Synonyms | Velcalcetide |
| Chemical data | |
| Formula | C38H73N21O10S2 |
| Molecular mass | 1,048.26 g·mol−1 |
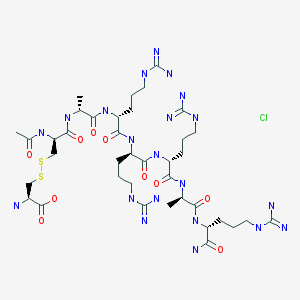
Etelcalcetide hydrochloride
RN: 1334237-71-6
UNII: 72PT5993DU
The term "AMG 416" refers to the compound having the chemical name: JV-acetyl-D- cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-D-arginamide disulfide with L- cysteine, which may be represented as:
H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2
The terms "AMG 416 hydrochloride" or "AMG 416 HQ" are interchangeable and refer to the compound having the chemical name: N-acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl- D-arginyl-D-alanyl-D-arginamide disulfide with L-cysteine hydrochloride, which may be represented as:
H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2 · xHCl
D-Argininamide, N-acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-, disulfide with L-cysteine, hydrochloride (1:?)
N-Acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-D-argininamide disulfide with L-cysteine hydrochloride
Amgen today announced the submission of a New Drug Application (NDA) with the United States Food and Drug Administration (FDA) for etelcalcetide (formerly AMG 416) for the treatment of secondary hyperparathyroidism (SHPT) in patients with chronic kidney disease (CKD) on hemodialysis. If approved, etelcalcetide will be the first calcimimetic agent that can be administered intravenously at the end of the dialysis session.
"Secondary hyperparathyroidism is a serious, progressive disease that can lead to significant clinical consequences and is also associated with a high pill burden for patients," said Sean E. Harper, M.D., executive vice president of Research and Development at Amgen. "We look forward to working with regulatory authorities during the review process to bring this important treatment to market, helping to fill an unmet need for the many patients impacted by this disease."
Etelcalcetide is a novel calcimimetic agent that suppresses the secretion of parathyroid hormone and is in clinical development for the treatment of SHPT in patients with CKD on hemodialysis. Etelcalcetide is administered intravenously three times per week at the end of each dialysis session. It acts by binding to and activating the calcium-sensing receptor on the parathyroid gland, thereby causing decreases in parathyroid hormone (PTH). Sustained elevations in PTH are known to be associated with significant clinical consequences for patients with CKD.
The submission includes data from three Phase 3 studies, all of which met the primary endpoints, including two pooled placebo-controlled trials in more than 1,000 patients and a head-to-head study evaluating etelcalcetide compared with cinacalcet.
About Secondary HyperparathyroidismSHPT is a common and serious condition that is often progressive among patients with CKD, and it affects many of the approximately two million people throughout the world who are receiving dialysis, including 450,000 people in the U.S. The disorder develops early in the course of CKD and usually manifests as increased levels of PTH as a result of increased production from the parathyroid glands (four small glands in the neck). Patients with end stage renal disease who require maintenance dialysis often have substantial elevations of PTH that are commonly associated with abnormal calcium and phosphorus levels and an increased risk of significant clinical consequences.
About Etelcalcetide (AMG 416)Etelcalcetide is a novel calcimimetic agent in clinical development for the treatment of SHPT in CKD patients on hemodialysis that is administered intravenously at the end of the dialysis session. Etelcalcetide binds to and activates the calcium-sensing receptor on the parathyroid gland, thereby decreasing PTH levels.
About Sensipar® (cinacalcet)Sensipar® (cinacalcet) is the first oral calcimimetic agent approved by the FDA for the treatment of SHPT in adult patients with CKD on dialysis. Sensipar is not indicated for use in adult patients with CKD who are not on dialysis because of an increased risk of hypocalcemia. The therapy is also approved in the U.S. for treatment of hypercalcemia in adult patients with parathyroid carcinoma and hypercalcemia in adult patients with primary HPT for whom parathyroidectomy would be indicated on the basis of serum calcium levels, but who are unable to undergo parathyroidectomy. Sensipar binds to the calcium-sensing receptor, resulting in a drop in PTH levels by inhibiting PTH synthesis and secretion. In addition, the reductions in PTH lower serum calcium and phosphorus levels.
.....................
WO 2011014707
..........................
WO 2014210489
A variety of compounds having activity for lowering parathyroid hormone levels have been described. See International Publication No. WO 2011/014707. In one embodiment, the compound may be represented as follows:
H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2
The main chain has 7 amino acids, all in the D-configuration and the side-chain cysteine residue is in the L-configuration. The amino terminal is acetylated and the carboxyl-terminal is amidated. This compound ("AMG-416") has utility for the treatment of secondary hyperparathyroidism (SHPT) in hemodialysis patients. A liquid formulation comprising AMG-416 may be administered to a subject intravenously. The hydrochloride salt of AMG-416 may be represented as follows:
H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2 · x(HCl)
Therapeutic peptides pose a number of challenges with respect to their formulation. Peptides in general, and particularly those that contain a disulfide bond, typically have only moderate or poor stability in aqueous solution. Peptides are prone to amide bond hydrolysis at both high and low pH. Disulfide bonds can be unstable even under quite mild conditions (close to neutral pH). In addition, disulfide containing peptides that are not cyclic are particularly prone to dimer formation. Accordingly, therapeutic peptides are often provided in lyophilized form, as a dry powder or cake, for later reconstitution. A lyophilized formulation of a therapeutic peptide has the advantage of providing stability for long periods of time, but is less convenient to use as it requires the addition of one or more diluents and there is the potential risk for errors due to the use of an improper type or amount of diluent, as well as risk of contamination. In addition, the lyophilization process is time consuming and costly.
Accordingly, there is a need for an aqueous liquid formulation comprising a peptide agonist of the calcium sensing receptor, such as AMG 416. It would be desirable for the liquid formulation to remain stable over a relevant period of time under suitable storage conditions and to be suitable for administration by intravenous or other parenteral routes.
.......................................
Milestones
- 25 Aug 2015Preregistration for Secondary hyperparathyroidism in USA (IV)
- 29 May 2015Pooled analysis efficacy and adverse events data from two phase III trials in secondary hyperparathyroidism released by Amgen
- 21 Apr 2015Amgen plans to submit Biological License Application to USFDA and Marketing Authorisation Application to EMA for Secondary hyperparathyroidism
References
- "Amgen Submits New Drug Application For Novel Intravenous Calcimimetic Etelcalcetide (AMG 416)”
- "Velcalcetide (AMG 416), a novel peptide agonist of the calcium-sensing receptor, reduces serum parathyroid hormone and FGF23 levels in healthy male subjects
- "Evidence for Chronic Kidney Disease-Mineral and Bone Disorder Associated With Metabolic Pathway Changes”
49th Congr Eur Renal Assoc - Eur Dialysis Transpl Assoc (May 24-27, Paris) 2012, Abst SAO054
KAI-4169, a novel peptide agonist of the calcium sensing receptor, attenuates PTH and soft tissue calcification and restores parathyroid gland VDR levels in uremic rats
49th Congr Eur Renal Assoc - Eur Dialysis Transpl Assoc (May 24-27, Paris) 2012, Abst SAO014
49th Congr Eur Renal Assoc - Eur Dialysis Transpl Assoc (May 24-27, Paris) 2012, Abst SAO014
Long term safety and efficacy of velcalcetide (AMG 416), a calcium-sensing receptor (CaSR) agonist, for the treatment of secondary hyperparathyroidism (SHPT) in hemodialysis (HD) patients
Kidney Week (November 5-10, Atlanta, GA) 2013, Abst SA-PO575
Kidney Week (November 5-10, Atlanta, GA) 2013, Abst SA-PO575
Preclinical PK and PD relationship for KAI-4169, a novel calcimimetic
93rd Annu Meet Endo Soc (June 4-7, Boston) 2011, Abst P1-198
93rd Annu Meet Endo Soc (June 4-7, Boston) 2011, Abst P1-198
KAI-4169, a novel calcimimetic for the treatment of secondary hyperparathyroidism
93rd Annu Meet Endo Soc (June 4-7, Boston) 2011, Abst P2-98
93rd Annu Meet Endo Soc (June 4-7, Boston) 2011, Abst P2-98
Characterization of KAI-4169, a novel peptide for the treatment of chronic kidney disease - Mineral and bone disorder, in a phase I study in healthy males
44th Annu Meet Am Soc Nephrol (ASN) (November 8-13, Philadelphia) 2011, Abst FR-PO1238
44th Annu Meet Am Soc Nephrol (ASN) (November 8-13, Philadelphia) 2011, Abst FR-PO1238
| WO2011014707A2 | Jul 29, 2010 | Feb 3, 2011 | Kai Pharmaceuticals, Inc. | Therapeutic agents for reducing parathyroid hormone levels |
////Etelcalcetide, Parathyroid Disease, Amgen Inc, AMG 416, KAI-4169; KAI-4169-HCl, ONO-5163, Telcalcetide, Velcalcetide, Velcalcetide hydrochloride