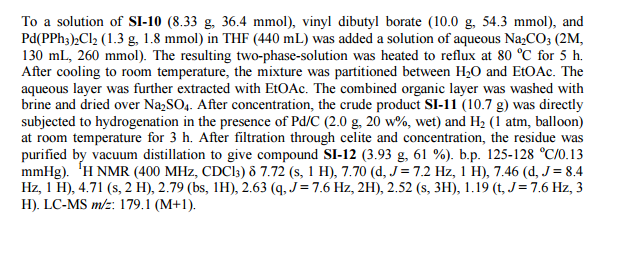
Siponimod , BAF-312
FREE FORM
| CAS Number: | 1230487-00-9 |
| Molecular Weight: | 516.59501 |
| Molecular Formula: | C29H35F3N2O3 |
1-[[4-[(E)-N-[[4-cyclohexyl-3-(trifluoromethyl)phenyl]methoxy]-C-methylcarbonimidoyl]-2-ethylphenyl]methyl]azetidine-3-carboxylic
acid
1-(4-{1-[(E)-4-cyclohexyl-3-trifluoromethylbenzyloxyimino]-ethyl}-2-ethylbenzyl)-azetidine-3-carboxylic acid
a selective modulator of S1P1 and S1P5 receptors,
allowing S1P1 receptor-dependent modulation of lymphocyte traffic
without producing S1P3 receptor-mediated effects.
Phase III
A sphingosine-1-phosphate receptor modulator potentially for the treatment of multiple sclerosis(MS).
Research Code BAF-312
CAS. 1230487-00-9, 1234627-85-0
As of January 2016 it is in a phase III clinical trial for secondary progressive MS due to complete Dec 2016.
AF312 is a potent and selective agonist of S1P with EC50 value of 0.39nM for S1P1 receptors and 0.98nM for S1P5 receptors, respectively [1]. BAF312 has shown >1000-fold selectivity for S1P1 versus S1P2, S1P3 and S1P4 receptors [1]. In vitro metabolism studies with liver microsomes have shown that the metabolic clearance of BAF312 is high in rat, low to moderate in monkey and human being, and low in dog and mouse. Moreover, BAF312 has been revealed to dose-dependently reduce peripheral lymphocyte counts in Lewis rats [2].For the detailed information about the solubility of BAF312 in water, the solubility of BAF312 in DMSO, the solubility of BAF312 in PBS buffer, the animal experiment of BAF312 ,the in vivo and in vitro test of BAF312 ,the cell experiment of BAF312 ,the IC50 and EC50 of BAF312

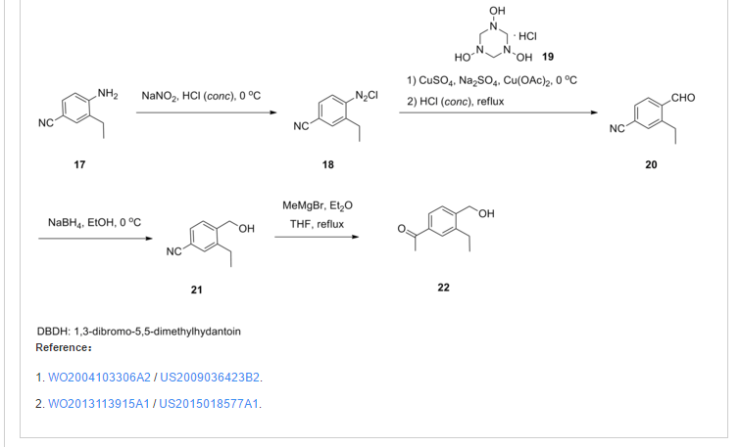
Clinical trials
(June 8, 2009) It is in Phase II trial. “A back-up compound for Fingolimod, BAF 312” is in Phase II studies.[2] It is being tested for the first time on people having multiple sclerosis. Worldwide 275 patients will participate in this phase II trial the outcome of which is to establish what the optimal dosage of BAF312 is for patients affected with Multiple Sclerosis for use in further trials. In order to identify “the optimal dosage”, participants in group I will be randomly selected to take either placebo, or BAF312 in doses of 0.5 mg/day, 2 mg/day, or 10 mg./day and will be regularly controlled in order to measure and determine the effectiveness, the tolerability and the safety of the dosages.A phase III trial should run from Dec 2012 to Dec 2016.[3]
Approvals and indications
None yetMechanism of action
Siponimod binds selectively to some of the Sphingosine-1-phosphate receptor forms – including Sphingosine-1-phosphate receptor 1 – found on lymphocytes and other cell types.This binding inhibits the migration of the lymphocytes to the location of the inflammation (e.g. in MS).
BAF312, may be very similar to Fingolimod but preventing lymphopenia, one of its main side effects, by preventing egress of lymphocytes from lymph nodes. BAF312 may be more selective in the particular sphingosine-1-phosphate receptors (8 in number) that it modulates.[4] It is selective for the -1 and -5 SIP receptors.[1]
MAIN
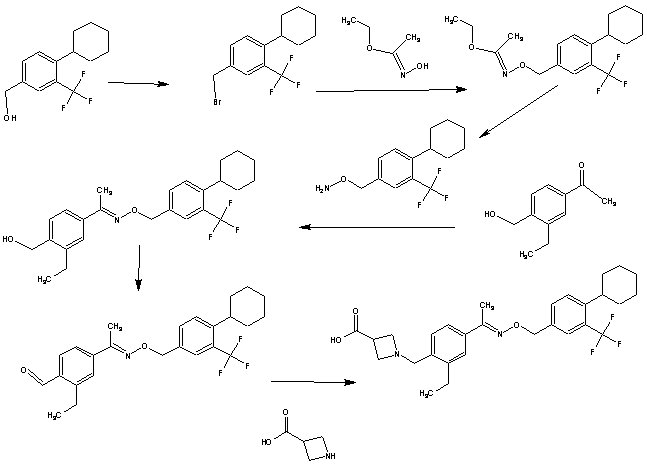
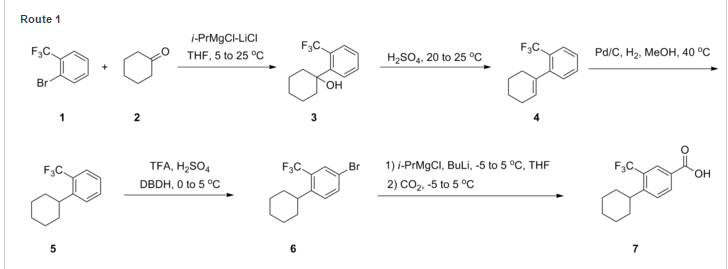
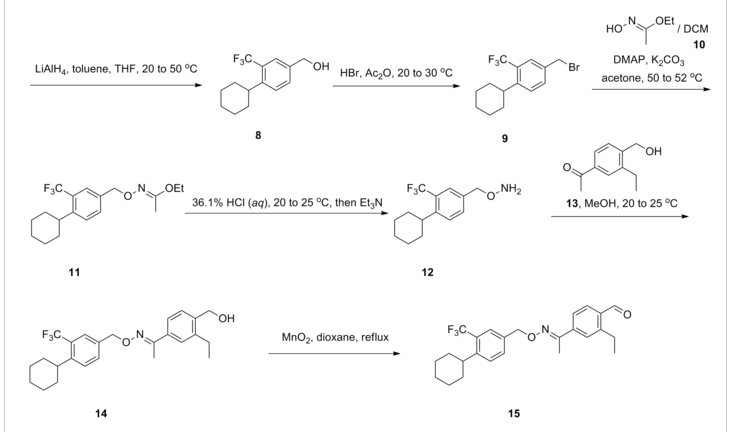
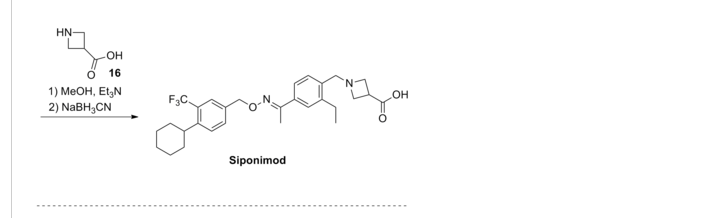
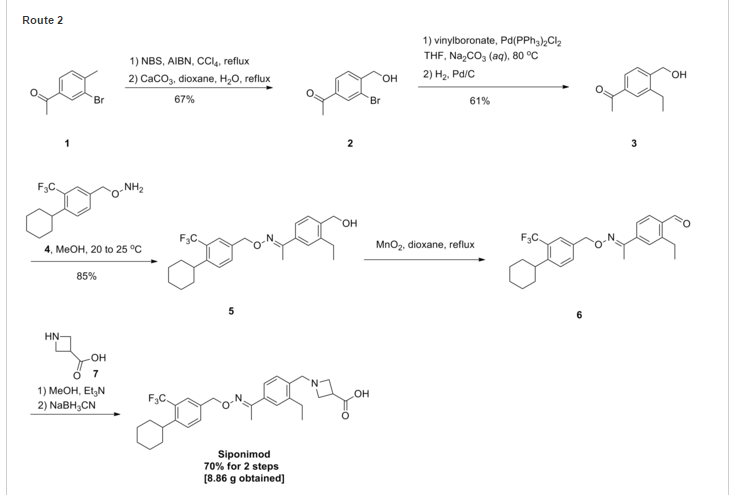
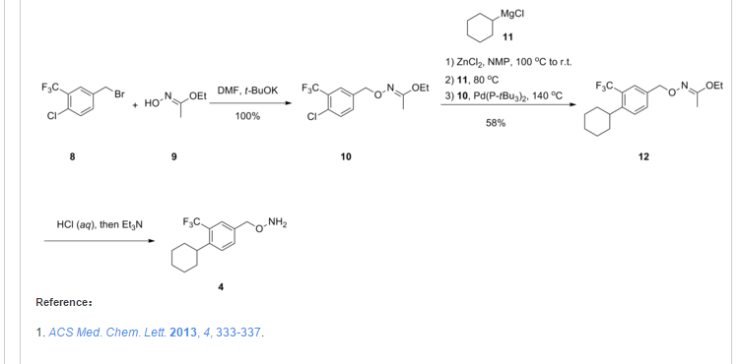
SYNTHESIS
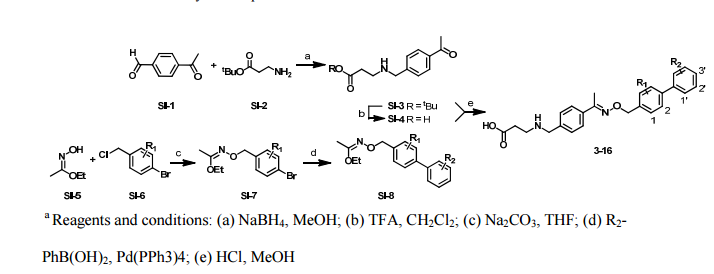
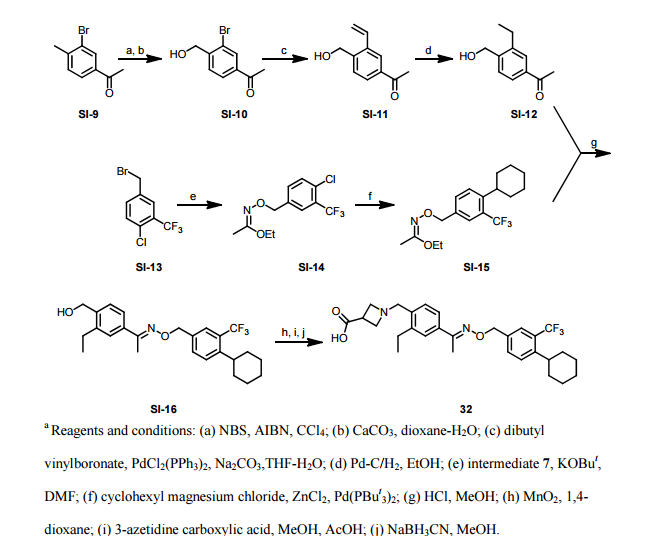
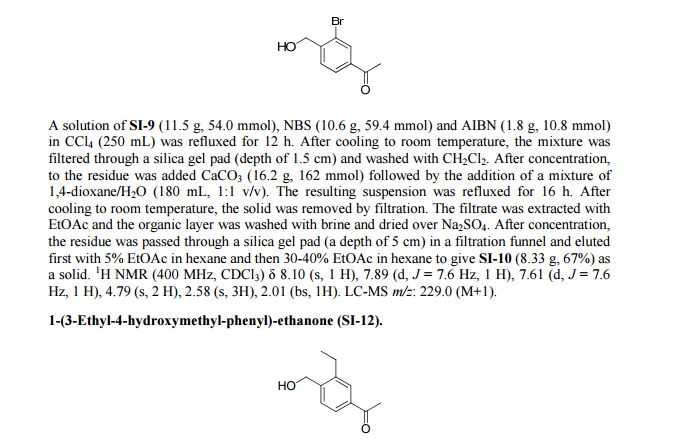
Paper
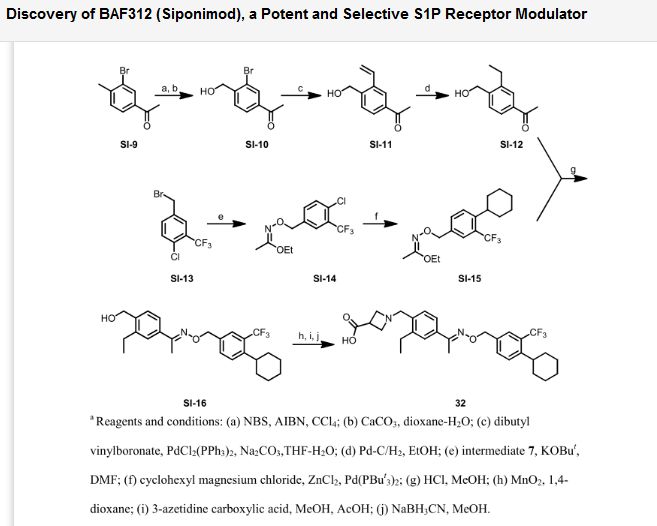
http://pubs.acs.org/doi/abs/10.1021/ml300396rDiscovery of BAF312 (Siponimod), a Potent and Selective S1P Receptor Modulator
† Genomics Institute of the Novartis Research Foundation, 10675 John Jay Hopkins Drive, San Diego, California 92121, United States
‡ Novartis Institute for Biomedical Research, Novartis Campus, CH-4056 Basel, Switzerland
ACS Med. Chem. Lett., 2013, 4 (3), pp 333–337
DOI: 10.1021/ml300396r
Publication Date (Web): January 04, 2013
Copyright © 2013 American Chemical Society
*Tel: 858-812-1621. E-mail: span@gnf.org.
Abstract

A novel series of alkoxyimino derivatives as S1P1
agonists were discovered through de novo design using FTY720 as the
chemical starting point. Extensive structure–activity relationship
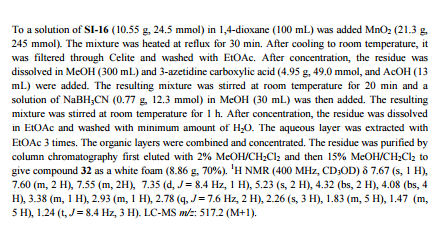
studies led to the discovery of (E)-1-(4-(1-(((4-cyclohexyl-3-(trifluoromethyl)benzyl)oxy)imino)ethyl)-2-ethylbenzyl)azetidine-3-carboxylic acid (32,
BAF312, Siponimod), which has recently completed phase 2 clinical
trials in patients with relapsing–remitting multiple sclerosis.
PATENT
EP-2990055-A1 / 2016-03-02
MEDICINAL COMPOSITION FOR INHIBITING FORMATION AND/OR ENLARGEMENT OF CEREBRAL ANEURYSM OR SHRINKING SAME
PATENT
US-9265754-B2 / 2016-02-23
Use of 1-{4-[1-(4-cyclohexyl-3-trifluoromethyl-benzyloxyimino)-ethyl]-2-ethyl-benzyl}-azetidine-3-carboxylic acid in treating symptoms associated with rett syndrome
PATENT
US-20160046573-A1 / 2016-02-18
IDENTIFYING PATIENT RESPONSE TO S1P RECEPTOR MODULATOR ADMINISTRATION
a fixed dose combination of BAF312 and a CYP2C9 metabolic activity promotor (e.g. rifampin or carbamezipine).
BAF312 is preferably administered at the standard
therapeutic dosage. The CYP2C9 metabolic activity promotor is preferably
administered at a dosage suitable to upregulate CYP2C9 to a level where
a reduced dosage of BAF312 is not considered clinically necessary.
1-{4-[1-(4-cyclohexyl-3-trifluoromethyl-benzyloxyimino)-ethyl]-2-ethyl-benzyl}-azetidine-3-carboxylic acid forms
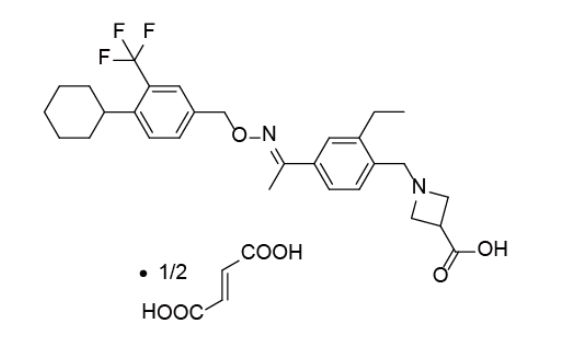
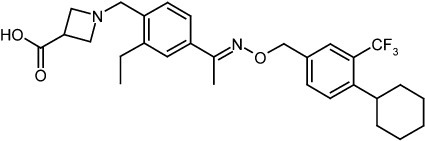
BAF312 (with the INN Siponimod) has the chemical name 1-{4-[1-(4-cyclohexyl-3-trifluoromethyl-benzyloxyimino)-ethyl]-2-ethyl-benzyl}-azetidine-3-carboxylic acid and has the structure of formula (I) below:
1-{4-[1-(4-cyclohexyl-3-trifluoromethyl-benzyloxyimino)-ethyl]-2-ethyl-benzyl}-azetidine-3-carboxylic acid
may be administered as a free base, as a pharmaceutically acceptable
salt (including polymorphic forms of the salt) or as a prodrug.
Pharmaceutically acceptable salt forms include hydrochloride, malate, oxalate, tartrate and hemifumarate.
In a preferred aspect, 1-{4-[1-(4-cyclohexyl-3-trifluoromethyl-benzyloxyimino)-ethyl]-2-ethyl-benzyl}-azetidine-3-carboxylic acid is administered as a hemifumarate salt.
PATENT
US-20150175536-A1 / 2015-06-25
HEMIFUMARATE SALT OF 1-[4-[1-(4-CYCLOHEXYL-3-TRIFLUOROMETHYL-BENZYLOXYIMINO)-ETHYL]-2-ETHYL-BENZYL]-AZETIDINE-3-CARBOXYLIC ACID
One particular compound disclosed in WO2004/103306 is 1-(4-{1-[(E)-4-cyclohexyl-3-trifluoromethyl-benzyloxyimino]-ethyl}-2-ethyl-benzyl)-azetidine-3-carboxylic acid (Compound I), the structure of which is shown below.
PATENT
EP-2809645-A1 / 2014-12-10
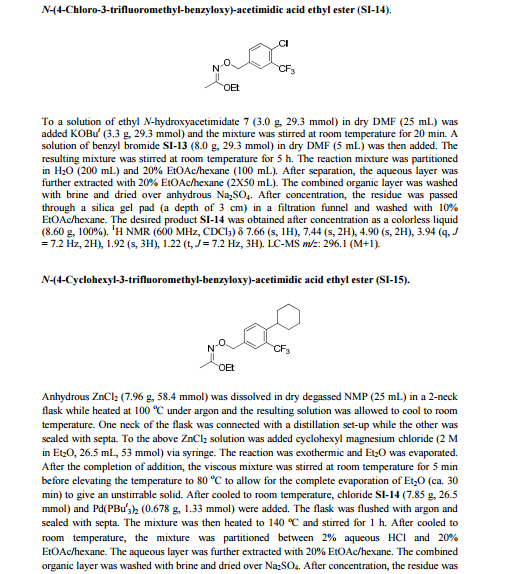
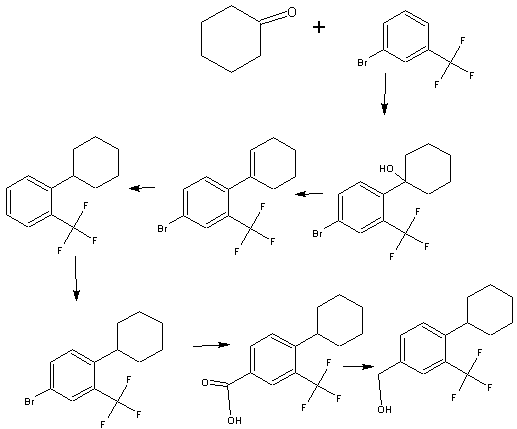
PROCESS FOR PREPARING N-(4-CYCLOHEXYL-3-TRIFLUOROMETHYL-BENZYLOXY)-ACETIMIDIC ACID ETHYL ESTER
PATENT
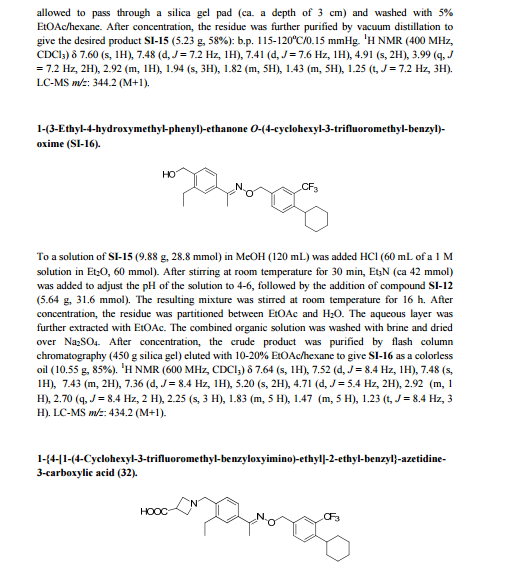
EP-2379498-B1 / 2015-01-21
POLYMORPHIC FORM OF 1-(4-{1-[(E)-4-CYCLOHEXYL-3-TRIFLUOROMETHYL-BENZYLOXYIMINO]-ETHYL}-2-ETHYL-BENZYL) -AZETIDINE-3-CARBOXYLIC ACID
Example 1 – Preparation of the Crystalline Form A of the free base of 1-(4-{1-[(E)-4-Cyclohexyl-3-trifluoromethyl-benzyloxyimino]-ethyl}-2-ethyl-benzyl)-azetidine-3-carboxylic acid (Compound I)Method
10 g of 1-4-{1-[(E)-4-Cyclohexyl-3-trifluoromethyl-benzyloxyimino]-ethyl}-2-ethyl-benzyldehyde, 4.7 g of 3-azetidine carboxylic acid and methanol (300 mL) are mixed. The resulting mixture is heated to 45 °C over 30 min and stirred at this temperature for 2 h. Then the reaction mixture is cooled to 20-25 °C and a solution of NaBH3CN (0.73 g) in MeOH (30 mL) is then added over a period of 20 min. The resulting mixture is stirred at room temperature for 1 h. After concentration, the residue is dissolved in EtOAc, (200 mL) and washed with minimum amount of H2O (20 mL). The organic layer is washed with water (2 x 10 mL) and concentrated to remove as much AcOH as possible. The residue is purified by column chromatography (minimum silica gel was used, 5 cm long by 3 cm diameter) first eluted with EtOAc and then MeOH to give 1-{4-[1-(4-Cyclohexyl-3-trifluoromethyl-benzyloxyimino)-ethyl]-2-ethyl-benzyl}-azetidine-3-carboxylic acid, as a thick oil. The residue is azeotroped with toluene to ca. 30 mL in volume, then heptane (60 mL) is added. The product crystallized after seeding with pure 1-{4-[1-(4-Cyclohexyl-3-trifluoromethyl-benzyloxyimino)-ethyl]-2-ethyl-benzyl}-azetidine-3-carboxylic acid. The suspension is stirred at 20-25 °C for 24 h and filtered. The filter cake is washed with toluene/heptane (1:3, 10 mL) and heptane (20 mL), and dried at 65 °C for 16 h. The product had a melting point of 110°C. 1H NMR (400 MHz, CD3OD) δ 7.67 (s, 1 H), 7.60 (m, 2 H), 7.55 (m, 2H), 7.35 (d, J = 8.4 Hz, 1 H), 5.23 (s, 2 H), 4.32 (bs, 2 H), 4.08 (bs, 4 H), 3.38 (m, 1 H), 2.93 (m, 1 H), 2.78 (q, J = 7.6 Hz, 2 H), 2.26 (s, 3 H), 1.83 (m, 5 H), 1.47 (m, 5 H), 1.24 (t, J = 8.4 Hz, 3 H).PATENT
WO2004/103306
Example 31 – (4-[ 1 -(4-Cvclohexyl-3-trifluoromethyl-benzyloxyimino)-ethyl]-2-ethyl-benzyll -azetidine-
3-carboxylic acid
To a suspension of MnO2 (10 eq) in dioxane is added l-(3-ethyl-4-hydroxymethyl- phenyl)-ethanone O-(4-cyclohexyl-3-trifluoromethyl-benzyl)-oxime (1 eq). The resulting mixture is refluxed for 10 minutes. After filtration and concentration, the residue is dissolved in MeOH and treated with azetidine-3-carboxylic acid (2 eq) and Et3N (1.5 eq). The resulting mixture is heated at 50°C for 30 minutes. After cooling to room temperature, NaBH3CN (3 eq) is added in portions. Purification by preparative LCMS results in l-{4-[l- (4-cyclohexyl-3-trifluoromethyl-benzyloxyimino)-ethyl]-2-ethyl-benzyl}-azetidine-3- carboxylic acid; Η NMR (400 MHz, CD3OD) δ 1.24 (t, 3H), 1.30-1.60 (m, 5H), 1.74-1.92 (m, 5H), 2.28 (s, 3H), 2.79 (q, 2H), 2.92 (m, 1H), 3.68 (m, 1H), 4.32 (m, 4H), 4.51 (s, 2H) 5.22 (s, 2H), 7.38 (d, 1H), 7.50-7.68 (m, 5H). MS: (ES+): 517.3 (M+l)+.
References
- 1Siponimod (BAF312) for the Treatment of Secondary Progressive Multiple Sclerosis: Design of the Phase 3 EXPAND Trial (P07.126)
- 2
- Multiple sclerosis and the pharmaceutical industry/Medicines in development for MS: abpi.org.uk 2009
- 3
- Exploring the Efficacy and Safety of Siponimod in Patients With Secondary Progressive Multiple Sclerosis (EXPAND)
- 4
WO 2008000419, Hiestand, Peter C; Schnell, Christian, “S1P Receptor modulators for treating multiple sclerosis”[
/////////BAF-312 , 1230487-00-9, 1234627-85-0 , Siponimod , BAF 312, Phase III , S1P receptor, S1P1 agonist, lymphocytes
N(CC1=CC=C(/C(=N/OCC2=CC=C(C3CCCCC3)C(C(F)(F)F)=C2)/C)C=C1CC)1CC(C(O)=O)C1