GSK1059615; 958852-01-2; GSK-1059615; UNII-07YMO87363;
- GSK 615
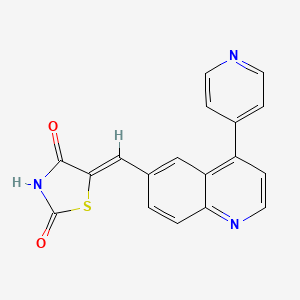
(5Z)-5-[(4-pyridin-4-ylquinolin-6-yl)methylidene]-1,3-thiazolidine-2,4-dione
5-[[4-(4-Pyridinyl)-6-quinolinyl]methylene]-2,4-thiazolidenedione
| C18H11N3O2S | |
| MOLECULAR WEIGHT: | 333.36384 |
|---|
CAS 958852-01-2
GSK1059615 is a potent, ATP-competitive inhibitor of PI 3-kinase alpha (PI3Kα) with IC50 of 2 nM. Phosphatidylinositol-3 kinases (PI3K) are critical for malignant cellular processes including growth, proliferation, and survival. GSK1059615 is also a novel inhibitor of PI3Kβ, PI3Kδ, PI3Kγ and mTOR with IC50 of 0.6 nM, 2 nM, 5 nM and 12 nM, respectively. GSK1059615 (25 mg/kg) effectively inhibits tumor growth in xenograft mice models of BT474 or HCC1954 breast cancer cells and attenuates MAPK signaling.
GSK1059615 is a phosphoinositide 3-kinase (PI3K) inhibitor with potential antineoplastic activity. PI3K inhibitor GSK1059615 inhibits PI3K in the PI3K/AKT kinase signaling pathway, which may trigger the translocation of cytosolic Bax to the mitochondrial outer membrane and an increase in mitochondrial membrane permeability, followed by apoptosis. Bax is a member of the proapoptotic Bcl-2 family of proteins. PIK3, an enzyme often overexpressed in cancer cells, plays a crucial role in tumor cell regulation and survival.
Patent
Example 1 (5Z)-5-{[4-(4-pyridinyl)-6-quinolinyl]methylidene}-1,3-thiazolidine-2,4-dione
a) 4-chloro-6-ethenylquinoline
A mixture of 6-bromo-4-chloroquinoline (6.52 g, 26.88 mmol; see J. Med. Chem., 21, 268 (1978)), tributyl(vinyl)tin (8.95 g, 28.22 mmol), and tetrakistriphenylphosphine palladium (0) (0.62 g, 0.54 mmol) in 1,4-dioxane (150 mL) was refluxed for 2.0 h, cooled to room temperature, and concentrated in vacuo. The residue was purified by flash chromatography on silica gel (0-4% MeOH:CH2Cl2) to give the title compound (5.1 g) as a pale yellow solid. MS (ES)+m/e 190 [M+H]+. This material was used directly in the next step.
b) 4-chloro-6-quinolinecarbaldehyde
A mixture of 4-chloro-6-ethenylquinoline (5.1 g, 26.88 mmol), 2,6-lutidine (5.76 g, 53.75 mmol), sodium (meta) periodate (22.99 g, 107.51 mmol), and osmium tetroxide (5.48 g of a 2.5% solution in tert-butanol, 0.538 mmol) in 1,4-dioxane:H2O (350 mL of 3:1 mixture) was stirred for 3.5 h at room temperature and concentrated in vacuo. The residue was purified by flash chromatography on silica gel (CH2Cl2) to give the title compound (4.26 g, 83% for 2 steps) as a pale yellow solid. MS (ES)+ m/e 192 [M+H]+.
c) 4-(4-pyridinyl)-6-quinolinecarbaldehyde
A mixture of 4-chloro-6-quinolinecarbaldehyde (3.24 g, 16.92 mmol), 4-pyridylboronic acid (3.12 g, 25.38 mmol), tetrakistriphenylphosphine palladium (0) (0.978 g, 0.846 mmol), and 2M aqueous K2CO3 (7.02 g, 50.76 mmol, 25.4 mls of 2M solution) in DMF (100 mL) was heated at 100° C. for 3.0 h and cooled to room temperature. The mixture was filtered through celite and the celite was washed with EtOAc. The filtrate was transferred to a separatory funnel, washed with water and saturated NaCl, dried (Na2SO4), filtered and concentrated in vacuo. The residue was purified by flash chromatography on silica gel (5% MeOH:CH2Cl2) to give the title compound (2.03 g, 51%) as a tan solid. MS (ES)+ m/e 235 [M+H]+.
d) (5Z)-5-{[4-(4-pyridinyl)-6-quinolinyl]methylidene}-1,3-thiazolidine-2,4-dione
A mixture of 4-(4-pyridinyl)-6-quinolinecarbaldehyde (0.108 g, 0.463 mmol), 2,4-thiazolidinedione (0.0417 g, 0.356 mmol), piperidine (0.0303 g, 0.356 mmol), and acetic acid (0.0214 g, 0.356 mmol) in EtOH (5 mL) was heated at 150° C. for 30 minutes in a microwave oven. The reaction was cooled to room temperature and the resulting precipitate was filtered and dried in a Buchner funnel to give the title compound (0.0594 g, 50%) as a tan solid. MS (ES)+ m/e 334 [M+H]+. 1H NMR (400 MHz, DMSO-d6) □ ppm 9.08 (d, J=4.42 Hz, 1H) 8.80-8.88 (m, 2H) 8.25 (d, J=8.72 Hz, 1H) 8.00-8.07 (m, 2H) 7.98 (s, 1H) 7.65-7.68 (m, 2H) 7.63 (d, J=4.42 Hz, 1H).
……………..
Schemes/Experimentals
Scheme I:
Conditions: a) Tributyl(vinyl)tin, Pd(PPh3)4, dioxane, reflux; b) OsO4, NaIO4, 2,6- lutidine, f-BuOH, dioxane, H2O, rt; c) heteroaryl (R) boronic acid, Pd(PPh3)4, 2 M K2CO3, DMF, 10O 0C; d) 2,4-thiazolidinedione, piperidine, AcOH, EtOH, μwave, 150 0C.
Examples:
Example 1 : (5Z)-5-ff4-(4-pyridinyl)-6-quinolinvnmethylidene}-1 ,3-thiazolidine-
2,4-dione
a) 4-chloro-6-ethenylquinoline
A mixture of 6-bromo-4-chloroquinoline (6.52 g, 26.88 mmol; see J. Med. Chem., 21_, 268 (1978) ), tributyl(vinyl)tin (8.95 g, 28.22 mmol), and tetrakistriphenylphosphine palladium (0) (0.62 g, 0.54 mmol) in 1 ,4-dioxane (150 ml.) was refluxed for 2.0 h, cooled to room temperature, and concentrated in vacuo. The residue was purified by flash chromatography on silica gel (0-4% MeOH:CH2CI2) to give the title compound (5.1 g) as a pale yellow solid. MS(ES)+ m/e 190 [M+H]+. This material was used directly in the next step.
b) 4-chloro-6-quinolinecarbaldehyde
A mixture of 4-chloro-6-ethenylquinoline (5.1 g, 26.88 mmol), 2,6-lutidine (5.76 g, 53.75 mmol), sodium (meta) periodate (22.99 g, 107.51 mmol), and osmium tetroxide (5.48 g of a 2.5% solution in tert-butanol, 0.538 mmol) in 1 ,4- dioxane:H2O (350 ml. of 3:1 mixture) was stirred for 3.5 h at room temperature and concentrated in vacuo. The residue was purified by flash chromatography on silica gel (CH2CI2) to give the title compound (4.26 g, 83% for 2 steps) as a pale yellow solid. MS(ES)+ m/e 192 [M+H]+.
c) 4-(4-pyridinyl)-6-quinolinecarbaldehyde
A mixture of 4-chloro-6-quinolinecarbaldehyde (3.24 g, 16.92 mmol), 4- pyridylboronic acid (3.12 g, 25.38 mmol), tetrakistriphenylphosphine palladium (0) (0.978 g, 0.846 mmol), and 2M aqueous K2CO3 (7.02 g, 50.76 mmol, 25.4 mis of 2M solution) in DMF (100 ml.) was heated at 1000C for 3.0 h and cooled to room temperature. The mixture was filtered through celite and the celite was washed with EtOAc. The filtrate was transferred to a separatory funnel , washed with water and saturated NaCI, dried (Na2SO4), filtered and concentrated in vacuo. The residue was purified by flash chromatography on silica gel (5% MeOHiCH2CI2) to give the title compound (2.03 g, 51%) as a tan solid. MS(ES)+ m/e 235 [M+H]+.
d) (5Z)-5-{[4-(4-pyridinyl)-6-quinolinyl]methylidene}-1 ,3-thiazolidine-2,4-dione
A mixture of 4-(4-pyridinyl)-6-quinolinecarbaldehyde (0.108 g, 0.463 mmol), 2,4-thiazolidinedione (0.0417 g, 0.356 mmol), piperidine (0.0303 g, 0.356 mmol), and acetic acid (0.0214 g, 0.356 mmol) in EtOH (5 ml.) was heated at 15O0C for 30 minutes in a microwave oven. The reaction was cooled to room temperature and the resulting precipitate was filtered and dried in a Buchner funnel to give the title compound (0.0594 g, 50%) as a tan solid. MS(ES)+ m/e 334 [M+H]+. 1 H NMR (400 MHz, DMSO-d6) D ppm 9.08 (d, J=4.42 Hz, 1 H) 8.80 – 8.88 (m, 2 H) 8.25 (d, J=8.72 Hz, 1 H) 8.00 – 8.07 (m, 2 H) 7.98 (s, 1 H) 7.65 – 7.68 (m, 2 H) 7.63 (d, J=4.42 Hz, 1 H).
| PATENT | SUBMITTED | GRANTED |
|---|---|---|
| THIAZOLIDINEDIONE DERIVATIVES AS PI3 KINASE INHIBITORS [US2008255115] | 2008-10-16 | |
| THIAZOLIDINEDIONE DERIVATIVES AS P13 KINASE INHIBITORS [US2009306074] | 2009-12-10 | |
| Role of PI3K p110 delta Signaling in Retroviral Infection and Replication [US2011135655] | 2011-06-09 | |
| PI3 KINASE INHIBITORS AND USES THEREOF [US2011230476] | 2011-09-22 |
Identification of druggable targets for radiation mitigation using a small interfering RNA screening assay.
Zellefrow CD,et al. Radiat Res. 2012 Sep;178(3);150-9. PMID: 22747550.
Zellefrow CD,et al. Radiat Res. 2012 Sep;178(3);150-9. PMID: 22747550.
Saadia et al (2009) Phosphatidylinositol-3-kinase as a therapeutic target in melanoma. Clin.Cancer Res. 15 3029. PMID: 19383818.
Knight et al (2010) Discovery of GSK2126458, a highly potent inhibitor of PI3K and the mammalian target of rapamycin. ACS Med.Chem.Lett. 1 39.
////////GSK 1059615, GSK 615