SKLB 1028
IND Filed
A multi-targeted inhibitor potentially for the treatment of leukemia and non small cell lung cancer.
SKLB-1028
CAS 1350544-93-2
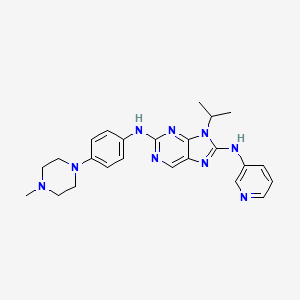
9-isopropyl-N2-(4-(4-methylpiperazin-1-yl)phenyl)-N8-(pyridin-3-yl)-9H-purine- 2,8-diamine
2-N-[4-(4-methylpiperazin-1-yl)phenyl]-9-propan-2-yl-8-N-pyridin-3-ylpurine-2,8-diamine
9-Isopropyl-N2-[4-(4-methylpiperazin-1-yl)phenyl]-N8-(3-pyridyl)-9H-purine-2,8-diamine, 443.5474, C24H29N9, Preclinical
9-isopropyl-N2-(4-(4-methylpiperazin-1-yl)phenyl)-N8-(pyridin-3-yl)-9H-purine- 2,8-diamine. Yield 65.6 %. HPLC>98.6%. 1H NMR(400 MHz, DMSO-d6): δ 9.22(s, 1H), 9.05(s, 1H), 8.94(d, J=2.8Hz, 1H), 8.39(s, 1H), 8.34(d,J=8.4Hz, 1H), 8.20(m, 1H), 7.63(d, J=8.8Hz, 2H), 7.37(m, 1H), 6.88 (d, J=8.8Hz, 2H), 4.88(m, 1H), 3.05(m, 4H), 2.45(m, 4H), 2.22(s, 3H), 1.69(s, 3H), 1.68(s, 3H)ppm。HRMS (ESI) m/z [M-H]- calcd for C24H29N9: 443.2546, found: 442.2538………..Leukemia (2012), 26(8)
PATENT
Synthetic route is as follows:


Example reaction is as follows:


8
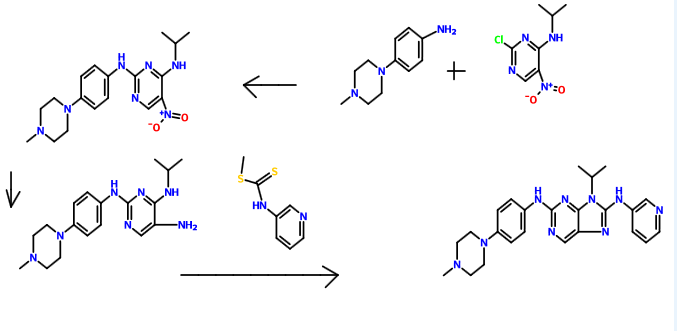
Preparation of chloro-4-amino-5-nitro pyrimidine of Example 12-

Was added dropwise 2,4-dichloro-5-nitro-pyrimidine (lO Aqueous ammonia (8.0ml) and Ν, Ν- diisopropylethylamine (13.2ml) was dissolved in 150ml dichloromethane, 0 ° C when .Og) in dichloromethane (30ml) solution, after dropwise, maintaining the temperature of the reaction one hour, the precipitate was filtered off, the filter cake was recrystallized to give a yellow solid 8.1g, yield 90.1%
Product 1HNMR (400MHz, DMSO-i¾): δ 9.20 (s, 1H), 9.02 (s, 1H), 8.60 (s, lH) ppm
Preparation of pyrimidine

Isopropylamine (4.5ml) and Ν, Ν- diisopropylethylamine (13.2ml) was dissolved in 150ml of dichloromethane, was added dropwise 2,4-dichloro-5-nitro-pyrimidine at 0 ° C ( lO.Og) in dichloromethane (30ml) solution, after dropwise, maintaining the reaction temperature for half an hour, and purified by column chromatography to give a light yellow solid was 10.1g, 90.4% yield of product 1H NMR (400 MHz, CDCl 3 ): [delta] 9.03 (s, 1H), 8.24 (s, 1H), 4.53 (m, 1H), 1.34 (d, J = 6.8 Hz, 6H) ppm 0
Example 16, 4-amino-2- (4- (4-methyl-piperazin-1-yl) anilino) -5-nitro-pyrimidin embodiment

4- (4-methylpiperazine) aniline (3.8g) was added to the compound 2-l (3.5g) in n-butanol (150ml) solution, the reaction for 4.5 hours at 90 ° C, cooled to room temperature, filtered , washed, and dried to give a red solid (5.2g), a yield of 79.5%. Product ‘H NMR (400 MHz, CDCl 3 ): [delta] 9.07 (s, 1H), 8.52 (s, 2H), 8.40 (s, 1H), 7.57 (s, 1H), 7.51 (s, 1H), 7.10 (m, 2H), 3.3 l (t, J = 4.8Hz, 4H), 2.81 (t, J = 4.8Hz, 4H), 2.30 (s, 3H) ppm.
Example 90,
9-isopropyl-2- (4- (4-methyl-piperazin-1-yl) anilino) -8- (pyridin-3-yl) -9H- purine
The compound 5- 7 (2.05g) was dissolved in dichloromethane (90ml), were added sequentially EDCI (2.3g), Ν, Ν- diisopropylethylamine (4.9ml), 3- pyridyl isothiocyanate ester (1.0g), stirred at room temperature for half an hour, then refluxed for 10 hours, TLC monitoring completion of the reaction the raw material 5-7 was cooled and purified by column chromatography to give a light red solid, yield 65.7%.

Product ESI-MS (m / z,%) 442.26 (MH) -. Ή NMR (400 MHz, DMSO-d 6 ): [delta] 9.38 (s, IH), 9.13 (s, IH), 8.99 (s, IH), 8.40 (s, IH), 8.36 (d, J = 8.4 Hz, IH), 8.20 (d, J = 4.4Hz, IH), 7.70 (d, J = 8.8Hz, 2H), 7.37 (m, IH), 6.96 (d, J = 8.8Hz, 2H), 4.97-4.92 ( m, IH), 3.35 (s, 6H), 2.80 (s, 3H): 2.53 (s, 2H), 1.69 (s, 6H) ppm.
/////////SKLB 1028, IND Filed, Preclinical
CN1CCN(CC1)c5ccc(Nc3nc4n(C(C)C)c(Nc2cccnc2)nc4cn3)cc5