PF-04995274,
(R)-4-((4-(((4-(Tetrahydrofuran-3-yloxy)-1,2-benzisoxazol-3-yl)oxy)methyl)piperidin-1-yl)methyl)tetrahydro-2H-pyran-4-ol
4-(4-{4-[(R)-(Tetrahydro-furan-3-yl)oxy]-benzo[d]isoxazol-3-yloxymethyl}-piperidin-1-ylmethyl)-tetrahydro-pyran-4-ol
CAS 1331782-27-4
UNII: XI179PG9LV
UNII: XI179PG9LV
MF C23-H32-N2-O6
MW 432.5138
a 5-HT4Partial Agonist
PHASE 1 Alzheimer’s type dementia.
Pfizer Inc. INNOVATOR
5-HT4 agonists have attracted attention for therapeutic value in the treatment of Alzheimer’s Disease (AD) and cognitive impairment.Acting to increase levels of acetylcholine and soluble APP alpha, 5-HT4 agonists have the potential to demonstrate both ameliorative and disease modifying effects
(R)-4-((4-((4-(tetrahydrofuran-3-yloxy)benzo[d]isoxazol-3-yloxy)methyl)piperidin-1-yl)methyl)tetrahydro-2/-/-pyran-4-ol and pharmaceutically acceptable salts thereof. This invention also is directed, in part, to a method for treating a 5-HT4 mediated disorder in a mammal. Such disorders include acute neurological and psychiatric disorders, stroke, cerebral ischemia, spinal cord trauma, head trauma, perinatal hypoxia, cardiac arrest, hypoglycemic neuronal damage, dementia, Alzheimer’s disease, Huntington’s Chorea, amyotrophic lateral sclerosis, ocular damage, retinopathy, cognitive disorders, idiopathic and drug- induced Parkinson’s disease, muscular spasms and disorders associated with muscular spasticity including tremors, depression, epilepsy, convulsions, migraine, urinary incontinence, substance tolerance, substance withdrawal, psychosis, schizophrenia, anxiety, mood disorders, trigeminal neuralgia, hearing loss, tinnitus, macular degeneration of the eye, gastroesophageal reflux disease, gastrointestinal disease, gastric motility disorder, non-ulcer dyspepsia, functional dyspepsia, irritable bowel syndrome, constipation, dyspepsia, esophagitis, gastroesophageral disease, nausea, emesis, brain edema, pain, tardive dyskinesia, sleep disorders, attention deficit/hyperactivity disorder, attention deficit disorder, disorders that comprise as a symptom a deficiency in attention and/or cognition, and conduct disorder
a(a) SOCl2, DMAP, acetone, DME, RT, 81%;
(b) DEAD, PPh3, THF, RT, 65%;
(c) K2CO3, MeOH, RT, 92%;
(d) K2CO3, water, MeOH, 50 °C, 76%;
(e) CDI, THF, 50 °C, 43%;
(f) DEAD, PPh3, THF, reflux, 51%;
(g) HCl, Et2O, RT, 81%;
(h) TEA, MeOH, reflux, 50%.

PAPER
Journal of Medicinal Chemistry (2012), 55(21), 9240-9254

The cognitive impairments observed in Alzheimer’s disease (AD) are in part a consequence of reduced acetylcholine (ACh) levels resulting from a loss of cholinergic neurons. Preclinically, serotonin 4 receptor (5-HT4) agonists are reported to modulate cholinergic function and therefore may provide a new mechanistic approach for treating cognitive deficits associated with AD. Herein we communicate the design and synthesis of potent, selective, and brain penetrant 5-HT4agonists. The overall goal of the medicinal chemistry strategy was identification of structurally diverse clinical candidates with varying intrinsic activities. The exposure–response relationships between binding affinity, intrinsic activity, receptor occupancy, drug exposure, and pharmacodynamic activity in relevant preclinical models of AD were utilized as key selection criteria for advancing compounds. On the basis of their excellent balance of pharmacokinetic attributes and safety, two lead 5-HT4 partial agonist candidates 2d and 3 were chosen for clinical development.
PATENT
(R)-4-((4-((4-(tetrahydrofuran-3-yloxy)benzo[d]isoxazol-3-yloxy)methyl)piperidin-1-yl)methyl)tetrahydro-2H-pyran-4-ol , hereinafter referred to as “Compound X,” and having the following structure:

Compound X
Example 1 : Synthesis of iR)-4-ii4-i(4-itetrahvdrofuran-3-yloxy)benzord1isoxazol-3-yloxy)methyl)piperidin-1 -yl)methyl)tetrahvdro- 2 -pyran-4-ol

Methyl 2-fluoro-6-hydroxybenzoate (2): To a 20L jacketed reactor were charged 2-fluoro-6-hydroxybenzoic acid (Oakwood Products; 0.972 kg, 6.31 mol), methanol (7.60 L) and sulfuric acid (0.710 kg, 7.24 mol, 1 .15 eq). The jacket temperature was heated to 60°C and the reaction mixture was stirred for 45 h. The reaction mixture was concentrated under vacuum and approximately 7.5 L of methanol distillates were collected. The resulting thin oil was cooled to 20°C. Water (7.60 L) and ethyl acetate (7.60 L) were charged to the reactor, and the product extracted into the organic layer. The EtOAc solution was washed with a solution of sodium bicarbonate (1.52 Kg) in water (6.92 L) followed by a brine solution of sodium chloride (1.74 kg) in water (4.08 L). The resulting EtOAc solution was concentrated to dryness. A light orange oil was isolated; the oil slowly crystallized upon standing to give the title compound (2) (0.952 Kg, 5.60 mol, 89% yield). 1 H NMR (400 MHz, CDCI3) δ ppm 3.97 (s, 3H), 6.59 (ddd, J=10.9, 8.2,1 .2, 1 H), 6.76 (dt, J=8.2, 1 .1 , 1 H), 7.35 (td, J=8.6, 6.3, 1 H), 1 1.24 (s, 1 H); 13C NMR (400 MHz, CDCI3) δ ppm 52.65, 102.56 (d, J=13), 106.90 (d, J=23), 1 13.31 (d, J=3.1 ), 135.34 (d, J=1 1 .5), 161 .02, 163.31 (d, J=62.2), 169.87 (d, 3.8); MS 171.045 (m+1 ). 2-Fluoro-N,6-dihydroxybenzamide (3): To a 50L reactor was charged water (4.47 L) and hydroxylamine sulfate (6.430 kg, 39.17 mol), the mixture was stirred at 25°C. A solution of potassium carbonate (3.87 Kg, 27.98 mol) in water (5.05 L) was slowly added to the reaction mixture to form a thick white mixture that was stirred at 20°C. A solution of methyl 2-fluoro-6-hydroxybenzoate (2) (0.952 Kg, 5.60 mol) in methanol (9.52 L) was slowly added to the reactor resulting in mild off gassing. The reaction mixture was then heated to 35°C and stirred for 20 h. The reaction mixture was cooled to 15°C and stirred for 1 h. The mixture was filtered to remove inorganic material. The reactor was rinsed with methanol (2.86 L) and the tank rinse was used to wash the inorganic cake.
Analysis of the cake indicated that it contained product. To a 20L reactor was charged methanol (10 L) and the inorganic cake and the mixture was stirred at 25°C for 30 min. The mixture was filtered and the cake washed with methanol (3 L).
The combined filtrates were charged back into the reactor and concentrated under vacuum with the jacket temperature set at 40°C until approximately 10 L remained. The mixture was held at 25°C and cone. HCI (5.51 L) was added. The reactor was cooled to 15°C and stirred for 2 h. The white slurry was filtered and the resulting product cake was washed with water (4.76L), blown dry with nitrogen and then dried in a vacuum oven at 40°C for 12 h. The desired product (3) (747 g, 4.36 mol), was isolated in 78% yield. 1 H NMR (400 MHz, CD3OD) δ ppm 4.91 (s, 3H), 6.63 (ddd, J=10.9, 8.5, 0.8, 1 H), 6.72 (dt, J=8.2, 0.8, 1 H), 7.31 (td, J=8.2, 6.6, 1 H); MS 172.040 (m+1 ).
4-Fluorobenzo[d]isoxazol-3-ol (4): To a 20L jacketed reactor were charged tetrahydrofuran (2.23 L) and 1 ,1 ‘-carbonyldiimidazole (0.910 Kg, 5.64 mol). The resulting mixture was stirred at 20°C. Then a solution of 2-fluoro-N,6-dihydroxybenzamide (3) (744 g, 4.34 mol) in tetrahydrofuran (4.45 L) was slowly charged to the reactor maintaining the temperature below 30°C and stirred at 25°C for 30 min during which some off gassing was observed. The reaction mixture was heated to 60°C over 30 min and stirred for 6 h. The reactor was cooled to 20°C followed by the addition of 1 N aqueous hydrogen chloride (7.48L) over 15 min to adjust the pH to 1. The jacket temperature was set to 35°C and the reaction mixture concentrated under vacuum to remove approximately 6.68L of THF. The reactor was cooled to 15°C and stirred for 1 h. The resulting white slurry was filtered, the cake was washed with water (3.71 L) and dried in a vacuum oven at 40°C for 12 h. The desired product, (4) (597 g, 3.90 mol), was isolated in 90% yield. 1 H NMR (400 MHz, CD3OD) δ ppm 4.93 (b, 1 H), 6.95 (dd, J=10.1 , 8.6, 1 H), (d, J=8.6, 1 H), 7.52-7.57 (m, 1 H); LRMS 154.029 (m+1 ).
Tert-butyl 4-(tosyloxymethyl)piperidine-1-carboxylate (5): To a 20L jacketed reactor were charged dichloromethane (8 L), N-boc-4-piperdine methanol (0.982 Kg, 4.56 mol) and p-toluenesulfonyl chloride (0.970 Kg, 5.09 mol) and the resulting mixture was stirred at 20°C for 5 min. Triethylamine (0.94 Kg, 9.29 mol) was added to the reactor via an addition funnel and the resulting deep red solution was stirred at 25°C for 16 h. A solution of sodium carbonate (0.96 Kg, 9.06 mol) in water (7.04 L) was charged to the reaction mixture and stirred for 1 h at 20°C. The phases were split and the organic layer washed with brine (6 L) and concentrated at 40°C to a low stir volume. Dimethylacetamide (2 L) was charged to the reactor and concentration continued under full vacuum at 40°C for 1 h. The solution of tert-butyl 4-(tosyloxymethyl)piperidine-l -carboxylate (5) in dimethyl acetamide was held for further processing. Yield was assumed to be 100% with approximately
90% potency. A sample was pulled and concentrated to dryness for purity analysis. 1 H NMR (400 MHz, CDCI3) δ ppm 1 .02-1 .12 (m, 2H), 1.14 (s, 9H), 1 .59-1.64 (m, 2H), 1.75-1.87 (m, 1 H), 2.43 (s, 3H), 2.55-2.75 (m, 2H), 3.83 (d, J=6.7, 2H), 3.95-4.20 (b, 2H), 7.33 (d, 8.6, 2H), 7.76 (d, 8.2, 2H); 13C NMR (400 MHz, CDCI3) δ ppm 21 .64, 28.15, 28.39, 35.74, 73.97, 79.50, 126.99, 127.84, 129.86, 132.84, 144.84, 154.63; LRMS 739.329 (2m+1 ).
Tert-butyl 4-((4-fluorobenzo[d]isoxazol-3-yloxy)methyl)piperidine-1-carboxylate (6): To a 20L jacketed reactor were charged dimethylacetamide (4.28 L), tert-butyl 4-(tosyloxymethyl)piperidine-1 -carboxylate (5) (1.68 Kg, 4.56 mol), 4-fluorobenzo[d]isoxazol-3-ol (4) (540 g, 3.51 mol), and potassium carbonate (960 g, 6.98 mol) resulting in a thick beige slurry. The reaction mixture was heated to 50°C and stirred for 20 h and then cooled to 20°C, followed by the addition of water (7.5 L) and ethyl acetate (5.37 L). After mixing for 15 min, the phases were settled and split. The organic layer was washed with water (5.37 L), sending the aqueous wash to waste. The organic mixture was distilled under vacuum with a maximum jacket temperature of 40°C until approximately 5 L remained in the reactor. Methanol (2.68 L) was added and the resulting solution concentrated under vacuum to about 3 L of a yellow oil. Methanol (2.68 L) was charged to the reactor and the resulting solution was stirred at 25°C for 15 min. Water (0.54 L) was added over 15 min resulting in a white slurry. The mixture was cooled to 15°C, stirred for 1 h and then filtered. The filter cake was washed with a solution of water (0.54 L) in methanol (2.14 L), then air dried for 30 min, transferred to a vacuum oven and dried at 40°C for 12 h. The desired product, (6) (746 g, 2.13 mol), was isolated in 61 % yield. 1 H NMR (400 MHz, CDCI3) δ ppm 1.23-1 .37 (m, 2H), 1 .45 (s, 9H), 1 .78-1 .88 (m, 2H), 2.04-2.17 (m, 1 H), 2.67-2.83 (m, 2H), 4.02-4.26 (m, 2H), 4.28 (d, 6.6, 2H), 6.89 (dd, J=8.6, 7.5, 1 H), 7.21 (d, J=9, 1 H), (td, 8.6, 4.9); LRMS 351.171 (m+1 ).
(R)-Tert-butyl 4-((4-(tetrahydrofuran-3-yloxy)benzo[d]isoxazol-3-yloxy)methyl)piperidine-1-carboxylate (8): To a 20 L glass reactor with the jacket set to 20°C were charged (R)-tetrahydrofuran-3-ol (7) (297 g, 3.37 mol) and dimethylacetamide (5.1 L). 2.0 M sodium bis(trimethylsilyl)amide in THF (1.37 L, 2.74 mol) was slowly added via an addition funnel while maintaining a pot temperature less than 30°C. The resulting orange/red solution was stirred at 25°C for 30 min. Then, tert-butyl 4-((4-fluorobenzo[d]isoxazol-3-yloxy)methyl)piperidine-1 -carboxylate (6) (640.15 g, 1.83 mol) was charged and the reaction mixture was stirred at 25°C for 16 h. The reaction mixture was cooled to 20°C and water (6.4 L) was slowly added over 45 min maintaining a pot temperature of less than 35°C. Ethyl acetate (6 L) was added and the biphasic mixture was stirred for 15 min and then separated. The aqueous layer was back extracted with additional ethyl acetate (4 L). The combined organics were then washed with water (5 L) and a 20% brine solution (5 L). The organic mixture was concentrated under vacuum with the jacket temperature set to 40°C to approximately 3 L and held for further processing. Quantitative yield of the desired product, (8) (0.76 Kg, 1 .82 mol), in ethyl acetate was assumed. A sample was pulled and concentrated to dryness for purity analysis. 1 H NMR (400 MHz, CDCI3) δ ppm 1 .25-1.38 (m, 2H), 1 .44 (s, 9H), 1.76-1 .84 (m, 2H), 1 .89-1.97 (b, 1 H), 1 .99-2.12 (m, 1 H), 2.14-2.28 (m, 2H), 2.63-2.84 (m, 2H), 3.90-4.21 (m, 6H), 4.24 (d, J=6.3, 2H), 5.00-5.05 (m, 1 H), 6.48 (d, J=8.2, 1 H), 6.98 (d, J=8.6, 1 H), 7.37 (t, J=8.2, 1 H); LRMS 419.216 (m+1 ).
(R)-3-(Piperidin-4-ylmethoxy)-4-(tetrahydrofuran-3-yloxy)benzo[d]isoxazole 4-methylbenzenesulfonate (9): To a 20L jacketed reactor charged ethyl acetate (6.1 L), (R)-tert-butyl 4-((4-(tetrahydrofuran-3-yloxy)benzo[d]isoxazol-3-yloxy)methyl)piperidine-1 -carboxylate (8) (0.76 kg, 1 .82 mol) and p-toluenesulfonic acid monohydrate (0.413 kg, 2.17 mol) and stirred at 20°C for 30 min. The reactor jacket was heated from 20 to 65°C over
1 h and then held at 65°C for 16 h. The reactor was cooled to 15°C over 1 h and granulated for 2 h. The resulting slurry was filtered, the cake was washed with EtOAc (3 L) and then air dried on the filter for 30 min. The cake was transferred to a vacuum oven and dried at 40°C for 12 h. The desired product, (9) (854 g, 1.74 mol), was isolated in 96% yield (two steps). 1 H NMR (400
MHz, CD3OD) δ ppm 1.54-1 .67 (m, 2H), 2.04-2.18 (m, 3H), 2.19-2.36 (m, 2H), 2.33 (s, 3H), 3.01 -3.12 (m, 2H), 3.41-3.50 (m, 2H), 3.86-4.01 (m, 4H), 4.26 (d, J=6.3, 2H), 4.90 (s, 2H), 5.14-5.19 (m, 1 H), 6.72 (d, J=8.2, 1 H), 7.02 (d, J=8.6, 1 H), 7.21 (d, J=7.8, 2H), 7.48 (t, J=8.6, 1 H), 7.70 (d, J=8.2, 2H); LRMS 319.165 (m+1 ).
(R)-4-((4-((4-(Tetrahydrofuran-3-yloxy)benzo[d]isoxazol-3-yloxy)methyl)piperidin-1-yl)methyl)tetrahydro-2H-pyran-4-ol (11): To a
20L jacketed reactor were charged water (7.5 L) and sodium carbonate (0.98 kg); the mixture was stirred at 20°C until all solids had dissolved. Then (R)-3-(piperidin-4-ylmethoxy)-4-(tetrahydrofuran-3-yloxy)benzo[d]isoxazole 4-methylbenzenesulfonate (9) (750 g, 1 .53 mol) and ethyl acetate (6.0 L) were added to the reactor and stirred at 20°C for 30 min. The phases were split and the lower aqueous layer was back extracted twice with ethyl acetate (6.0 L and then 3.75 L). The organic layers were combined in the 20L reactor and washed twice with brine (3.0 L). The ethyl acetate solution was concentrated to under vacuum at 45°C to a low stir volume. Isopropyl alcohol (3.75 L) was added and concentration continued until 2 L remained in the reactor.
Additional isopropyl alcohol (2.75 L) was added and the mixture cooled to 25°C. To the reactor was charged 1 ,6-dioxaspiro[2.5]octane (10) (260 g, 2.29 mol) and the resulting solution heated to 50°C and stirred for 16 h. The reaction mixture was cooled to 30°C and water (15 L) was added over 60 min. Product crystallized from solution and the resulting slurry was cooled to 15°C over 1 h and then granulated for 4 h. The product was filtered and washed with water (3.75 L). The cake was blown dry with nitrogen for 30 min and then transferred to a vacuum oven and dried at 40°C for 12 h. The desired product, (11 ) (588 g, 1 .36 mol), was isolated in 89% yield.
1 H NMR (400 MHz, CDCI3) δ ppm 1 .41-1 .63 (m, 6H), 1.71 -1.81 (m, 2H), 1.81 -1.94 (m, 1 H), 2.17-2.26 (m, 2H), 2.33 (s, 2H), 2.4 (td, J=1 1.7, 2.3, 2H), 2.92 (d, J=1 1 .8, 2H), 3.46 (s, 1 H), 3.71-3.84 (m, 4H), 3.91 -4.10 (m, 4H), 4.24 (d, J=5.9, 2H), 5.03-5.08 (m, 1 H), 6.50 (d, J=8.2, 1 H), 7.00 (d, J=8.2, 1 H), 7.38 (t, J=8.2, 1 H);
13C NMR (400 MHz, CDCI3) δ ppm 29.1 1 , 33.10, 35.20, 36.92, 36.96, 56.15, 63.93, 67.14, 67.46, 68.27, 72.94, 74.06, 78.37, 103.17, 105.15, 131.71 , 152.71 , 166.02, 166.28;
LRMS 433.232 (m+1 ).
Example 2: Synthesis of iR)-4-ii4-i(4-itetrahvdrofuran-3-yloxy)benzord1isoxazol-3-yloxy)methyl)piperidin-1 -yl)methyl)tetrahvdro- 2H-pyran-4-ol

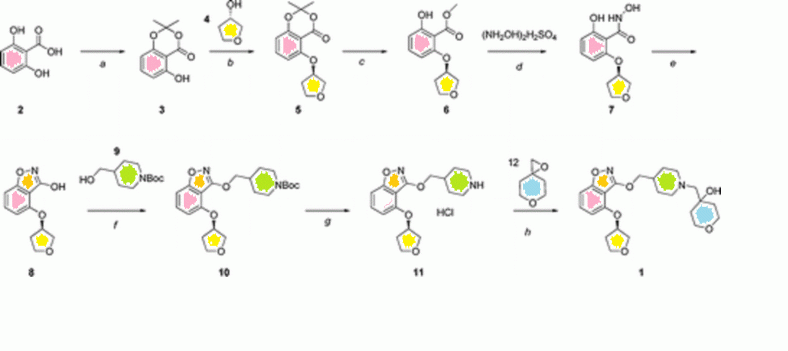
5-Hydroxy-2,2-dimethyl-benzo[1,3]dioxin-4-one: Thionyl chloride (83.8 g, 0.71 mol) was slowly added to a solution of 2,6-dihydroxy-benzoic acid (77 g, 0.5 mol), acetone (37.7 g, 0.65 mol) and DMAP (3.1 g, 0.025 mol) in dimethoxyethane (375 mL). The mixture was stirred at RT for 7 h. The residue obtained after concentration under reduced pressure was dissolved in ethyl
acetate and washed with water and aqueous saturated sodium bicarbonate solution. The organic layer was dried (Na2S04) and concentrated to afford 79 g desired product as a red solid (81 % yield). 1 H NMR (400 MHz, CDCI3) δ ppm 1 .68 (s, 6H), 6.37 (dd, J=8, 0.8, 11-1) 6.56 (dd, J=8, 0.8, 1 H), 7.34 (t, J=8, 1 H), 10.27( brs, 1 H).
2,2-Dimethyl-5-[(R)-(tetrahydro-furan-3-yl)oxy]-benzo[1,3]dioxin-4-one:
Diethyl azodicarboxylate (130.5 g, 0.75 mol) was added in a dropwise fashion to a mixture of 5-hydroxy-2,2-dimethyl-benzo[1 ,3]dioxin-4-one (100 g, 0.51 mol), triphenylphosphine (196.5 g, 0.75 mol), and (S)-tetrahydro-furan-3-ol (44 g, 0.5 mol) in 600 ml. of anhydrous THF. The resulting mixture was stirred at RT for 18 h. The solvent was removed under reduced pressure and the crude material was purified on a silica gel flash column, eluting with petroleum ether/ ethyl acetate (15:1 -> 3:1 ). 86 g (65% yield) of product was isolated as a colorless oil. 1 H NMR (400 MHz, CDCI3) δ ppm 1.67 (s, 6H), 2.30 (m, 2H), 4.2 (m, 4H) 4.97 (m, 1 H), 6.49 (d, J=8.4, 1 H) 6.51 (d, J=8.4, 1 H), 7.39 (t,
J=8.4, 1 H).
2-Hydroxy-6-[(R)-(tetrahydro-furan-3-yl)oxy]-benzoic acid methyl ester: Potassium carbonate (134.8 g, 0.98 mol) was added to a solution of 2,2-dimethyl-5-[(R)-(tetrahydro-furan-3-yl)oxy]-benzo[1 ,3]dioxin-4-one (86 g, 0.33 mol) in 1 L methanol. The mixture was stirred at RT for 2 h, then concentrated in vacuo. The residue was dissolved in ethyl acetate and washed with aqueous ammonium chloride solution. The organic layer was dried (Na2S04) and concentrated to afford 72 g of the product as a yellow solid (92% yield). 1 H NMR (400 MHz, CDCI3) δ ppm 2.20 (m, 2H), 3.99 (s, 3H), 4.80(m, 4H). 4.94 (m, 1 H), 6.31 (dd, J=8.4, 0.8, 1 H), 6.59 (dd, J=8.4, 0.8, 1 H), 7.30 (t, J=8.4, 1 H).
2,N-Dihydroxy-6-[(R)-(tetrahydro-furan-3-yl)oxy]-benzamide: Potassium carbonate (121 g. 0.867mmol) was added portionwise to a solution of hydroxylamine sulfate (120 g, 0.732 mol) in 360 ml. of water at 0°C. After stirring for 30 min, sodium sulfite (3.74 g, 0.029 mol) and a solution of 2-hydroxy-6-[(R)-(tetrahydro-furan-3-yl)oxy]-benzoic acid methyl ester (35 g, 0.146 mol) in 360 ml. of methanol were added and the mixture was stirred at 50°C for 30 h. Methanol was removed from the cooled reaction mixture under reduced pressure and the resulting aqueous layer was acidified with 2N HCI. The aqueous layer was extracted with ethyl acetate and the organic layer was dried (Na2S04) and concentrated to afford 25 g (76% yield ) of the product as a yellow solid. 1 H NMR (400 MHz, CDCI3) δ ppm 2.00 (m, 1 H), 2.15 (m, 1 H), 3.80 (m, 4H), 5.05 (m, 1 H), 6.48 (d, J=8, 1 H), 6.49 (d, J=8, 1 H), 7.19 (t, J=8, 1 H), 10.41 (brs, 1 H), 1 1.49 (brs, 1 H); LRMS m/z 239 (m+1 ).
4-[(R)-(Tetrahydro-furan-3-yl)oxy]-benzo[d]isoxazol-3-ol: A solution of 2, N-dihydroxy-6-[(R)-(tetrahydro-furan-3-yl)oxy]-benzamide (25 g, 0.105 mol) in 250 ml. of THF was heated to 50°C. Carbonyl diimidazole was added portionwise and the resulting mixture was stirred at 50°C for 14 h. After cooling to RT, 100 ml. of 2N HCI was added and the aqueous layer was extracted with ethyl acetate. The combined organic layers were then extracted three times with 10% aqueous potassium carbonate. The potassium carbonate aqueous extracts were washed with ethyl acetate and then acidified to pH 2 – 3 with 2N HCI. The acidified aqueous layer was extracted with ethyl acetate. The ethyl acetate extracts were washed with brine, dried (Na2S04) and concentrated to afford 20 g of product as a yellow solid (43% yield). 1 H NMR (400 MHz, CDCI3) δ ppm 2.20 (m, 2H), 3.89 (m, 1 H), 4.01 (m, 3H), 5.05 (m, 1 H), 6.48 (d, J=7.6, 1 H). 6.92 (d, J=7.6, 1 H), 7.37 (t, J=7.6, 1 H); LRMS m/z 222 (m+1 ).
4-{4-[(R)-(Tetrahydro-furan-3-yl)oxy]-benzo[d]isoxazol-3-yloxymethyl}-piperidine-1-carboxylic acid tert-butyl ester: Diethyl azodicarboxylate (15.6 g, 0.09 mol) was added to a mixture of 4-[(R)-(tetrahydro-furan-3-yl)oxy]-benzo[d]isoxazol-3-ol (10 g, 0.045 mol), 4-hydroxymethyl-piperidine-1 -carboxylic acid tert-butyl ester (1 1.6 g, 0.054 mol) and triphenylphosphine (23.5 g, 0.09 mol) in 300 mL THF. After the addition was complete the mixture was heated at reflux for 18 h. After concentration in vacuo, the crude product was purified on a silica gel flash column, eluting with petroleum ether/ ethyl acetate (15:1 -» 5:1 ) to afford 22 g of the product as an oil (51 % yield). 1 H NMR (400 MHz, CDCI3) δ ppm 1.25 (m, 2H), 1.39 (s, 9H), 1.76 (m, 2H), 1.99 (m, 1 H). 2.15 (m, 2H), 2.70 (bt, J=1 1.6, 2H), 3.95 (m, 4H). 4.13 (m, 2H). 4.34 (d J=6.4, 2H), 4.98 (m, 1 H), 6.43 (d, J=8, 1 H), 6.93 (d, J=8, 1 H), 7.31 (t, J=8, 1 H).
3-(Piperidin-4-ylmethoxy)-4-[(R)-(tetrahydro-furan-3-yl)oxy]-benzo[d]isoxazole: A 0°C solution of 4-{4-[(R)-(tetrahydro-furan-3-yl)oxy]-benzo[d]isoxazol-3-yloxymethyl}-piperidine-1 -carboxylic acid tert-butyl ester in 500 mL ether was treated with a saturated solution of HCI (g) in 200 mL ether. After addition was complete, the mixture was warmed to RT and stirred for 16 h. The reaction mixture was filtered. The white solid was washed with ethyl acetate followed by ether and dried to yield 15 g (81 % yield) of the desired product as a white solid. 1 H NMR (400 MHz, CD3OD) 5 ppm 1 .51 – 1.69 (m, 2 H) 2.04 – 2.19 (m, 3 H) 2.22 – 2.37 (m, 2 H) 2.99 – 3.14 (m, 2 H) 3.40 – 3.51 (m, 2 H) 3.85 – 4.02 (m, 4 H) 4.25 – 4.31 (m, 2 H) 5.17 (td, J= >1^ , 1 .56 Hz, 1 H) 6.72 (d, J=8.00 Hz, 1 H) 7.01 (d, J=8.59 Hz, 1 H) 7.47 (t, J=8.20 Hz, 1 H); LRMS m/z 319 (m+1 ).
4-(4-{4-[(R)-(Tetrahydro-furan-3-yl)oxy]-benzo[d]isoxazol-3-yloxymethyl}-piperidin-1-ylmethyl)-tetrahydro-pyran-4-ol: 1 ,6-Dioxa-spiro[2.5]octane (Focus Synthesis; 9.7 g, 0.084 mol) and triethylamine (8.6 g, 0.084 mol) were added to a solution of 3-(piperidin-4-ylmethoxy)-4-[(R)-(tetrahydro-furan-3-yl)oxy]-benzo[d]isoxazole (15 g, 0.042 mol) in 200 mL methanol. The resulting solution was heated at reflux for 18 h. The cooled mixture was concentrated and ethyl acetate and water were added to the residue. The layers were separated and the organic extracts were washed with brine, dried (Na2S04) and concentrated to provide 17 g crude product as a yellow oil. The crude material was purified by prep HPLC to afford 10 g of the desired product as a white solid. (50% yield).
1 H NMR (400 MHz, CDCI3) δ ppm 1.41 -1.63 (m, 6H), 1.71-1.81 (m, 2H), 1 .81 -1 .94 (m, 1 H), 2.17-2.26 (m, 2H), 2.33 (s, 2H), 2.4 (td, J=1 1 .7, 2.3, 2H), 2.92 (d, J=1 1.8, 2H), 3.46 (s, 1 H), 3.71-3.84 (m, 4H), 3.91-4.10 (m, 4H), 4.24 (d, J=5.9, 2H), 5.03-5.08 (m, 1 H), 6.50 (d, J=8.2, 1 H), 7.00 (d, J=8.2, 1 H), 7.38 (t, J=8.2, 1 H);
13C NMR (101 MHz, CDCI3) δ ppm 29.1 1 , 33.10, 35.20, 36.92, 36.96, 56.15, 63.93, 67.14, 67.46, 68.27, 72.94, 74.06, 78.37, 103.17, 105.15, 131.71 , 152.71 , 166.02, 166.28.
PAPER
Two Routes to 4-Fluorobenzisoxazol-3-one in the Synthesis of a 5-HT4Partial Agonist
† Groton Laboratories, Worldwide Research & Development, Pfizer Inc., Eastern Point Road, Groton, Connecticut 06340,United States
‡ Porton Fine Chemical, 1 Fine Chemical Zone, Chongqing Chemical Industrial Park, Changshou, Chongqing 401221China
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.5b00389
Publication Date (Web): February 2, 2016
Copyright © 2016 American Chemical Society
*E-mail: daniel.w.widlicka@pfizer.com.

A potent 5-HT4 partial agonist, 1 (PF-04995274), targeted for the treatment of Alzheimer’s disease and cognitive impairment, has been prepared on a multi-kilogram scale. The initial synthetic route, that proceeded through a 4-substituted 3-hydroxybenzisoxazole core, gave an undesired benzoxazolinone through a Lossen-type rearrangement. Route scouting led to two new robust routes to the desired 4-substituted core. Process development led to the efficient assembly of the API on a pilot plant scale under process-friendly conditions with enhanced throughput. In addition, crystallization of a hemicitrate salt of the API with pharmaceutically beneficial properties was developed to enable progression of clinical studies.
REFERNCES
Noguchi, H.; Waizumi, N. Preparation of benzisoxazole derivatives for treatment of 5-HT4 mediated disorders. PCT Int. Appl. WO/2011/101774 A1, 20110825
////////PF-04995274, PF 04995274, PFIZER, Alzheimer’s type dementia, PHASE 1
c1cc2c(c(c1)O[C@@H]3CCOC3)c(no2)OCC4CCN(CC4)CC5(CCOCC5)O