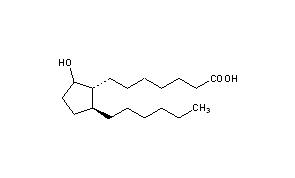
Rosaprostol
CAS Registry Number: 56695-65-9
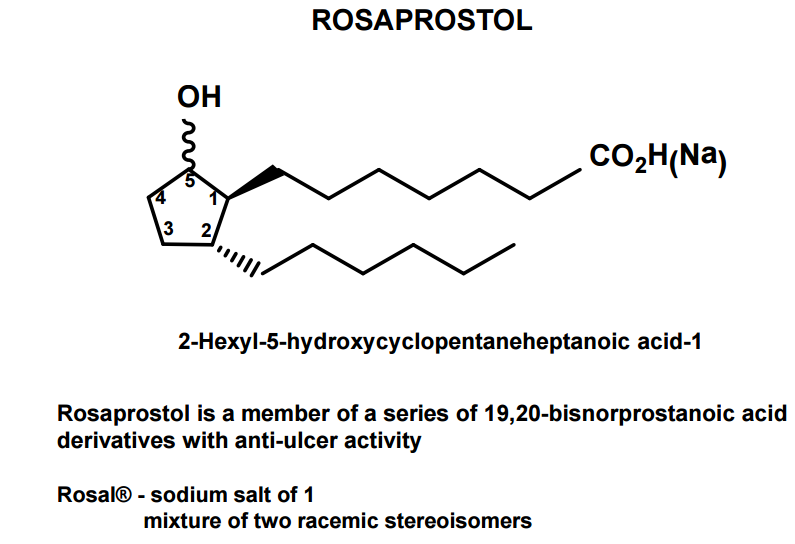
CAS Name: 2-Hexyl-5-hydroxycyclopentaneheptanoic acid
Additional Names: 9-hydroxy-19,20-bisnorprostanoic acid
Manufacturers' Codes: C-83; IBI-C83
Trademarks: Rosal (IBI)
Molecular Formula: C18H34O3
Molecular Weight: 298.46
Percent Composition: C 72.44%, H 11.48%, O 16.08%
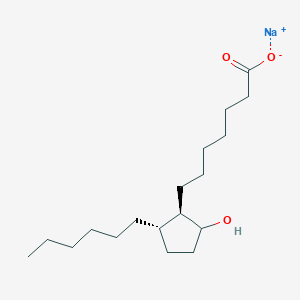
Derivative Type: Sodium salt
CAS Registry Number: 56695-66-0
Molecular Formula: C18H33NaO3
Molecular Weight: 320.44
Percent Composition: C 67.47%, H 10.38%, Na 7.17%, O 14.98%
Properties: White solid. LD50 orally in mice: ~3000 mg/kg (Valcavi, 1978); orally in rats: >5 g/kg (Valcavi, 1982).
Toxicity data: LD50 orally in mice: ~3000 mg/kg (Valcavi, 1978); orally in rats: >5 g/kg (Valcavi, 1982)
Therap-Cat: Antiulcerative.
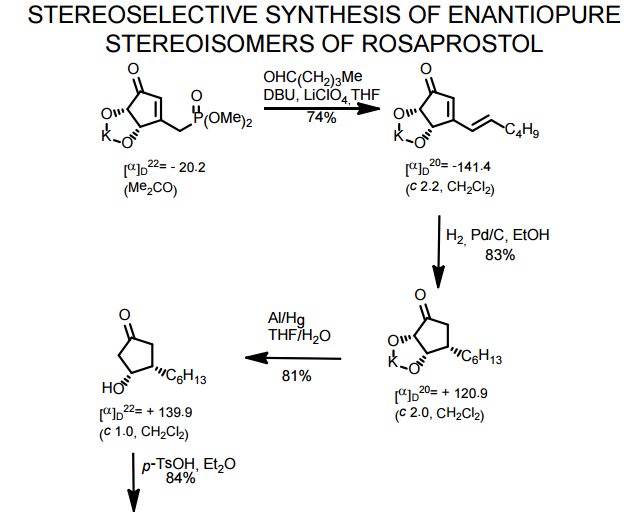
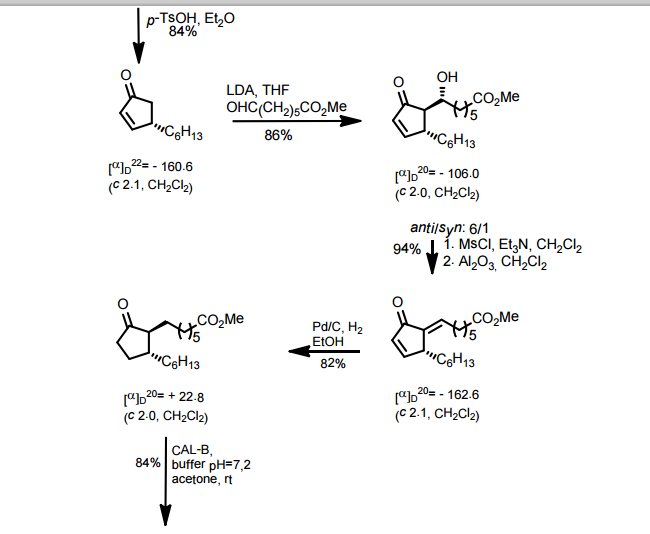
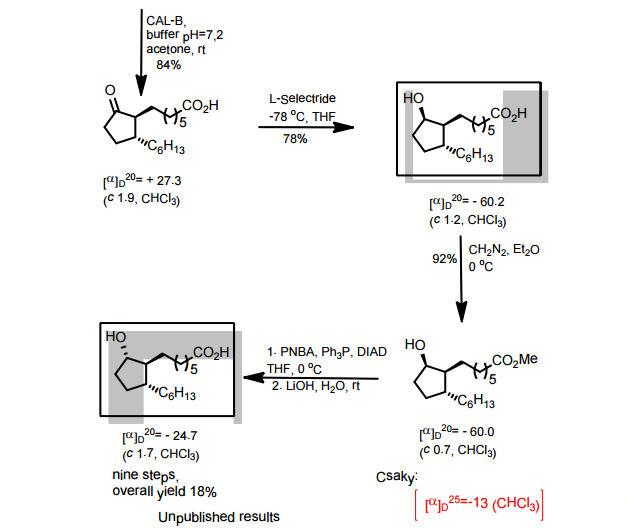
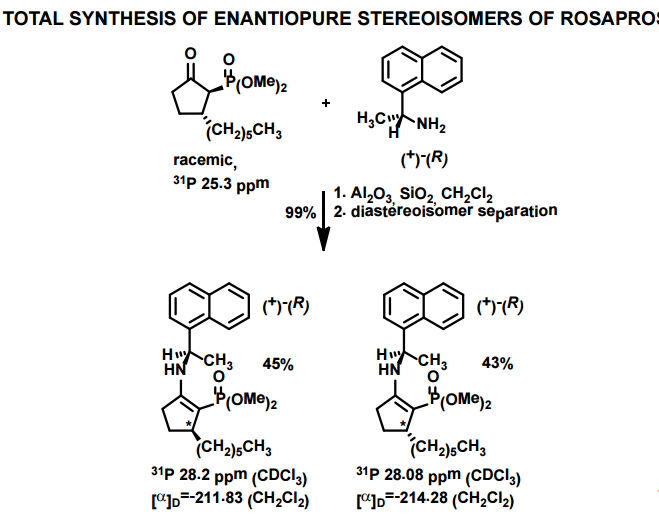
J. Org. Chem. 1998, 63, 8894-8897

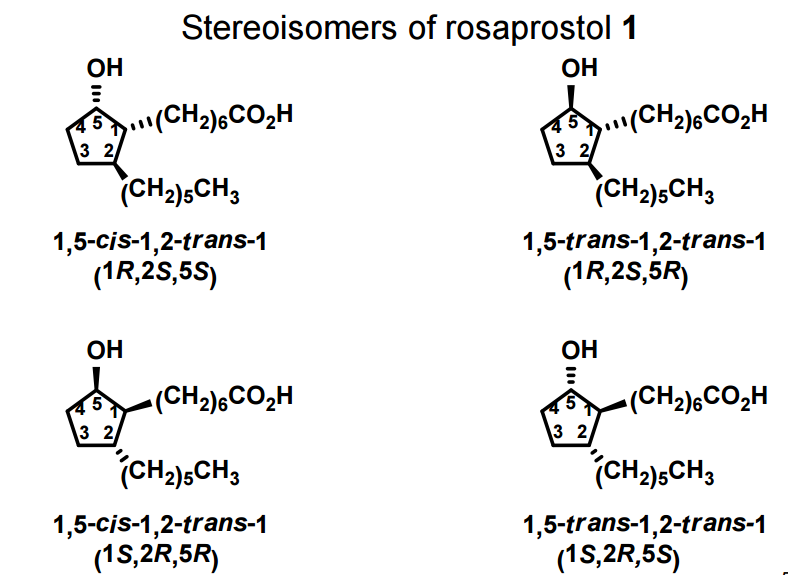
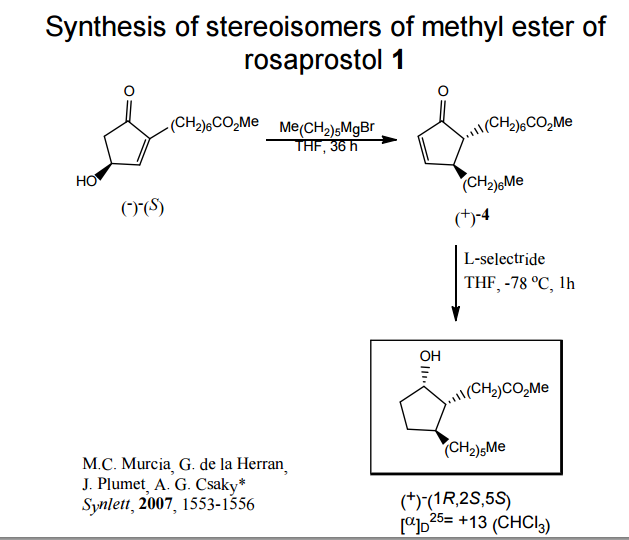
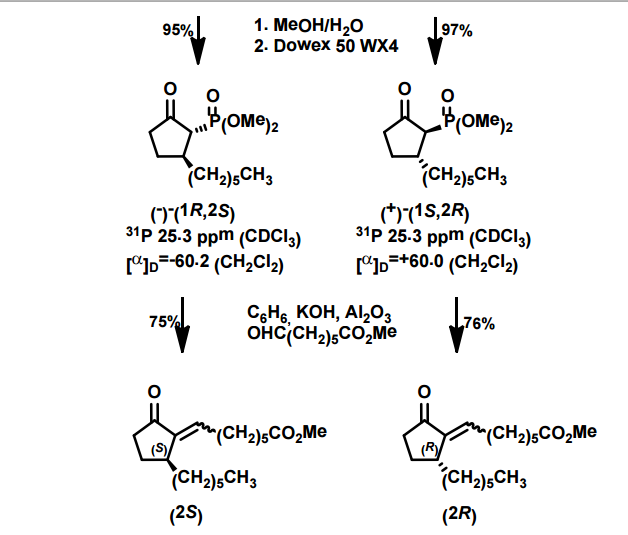
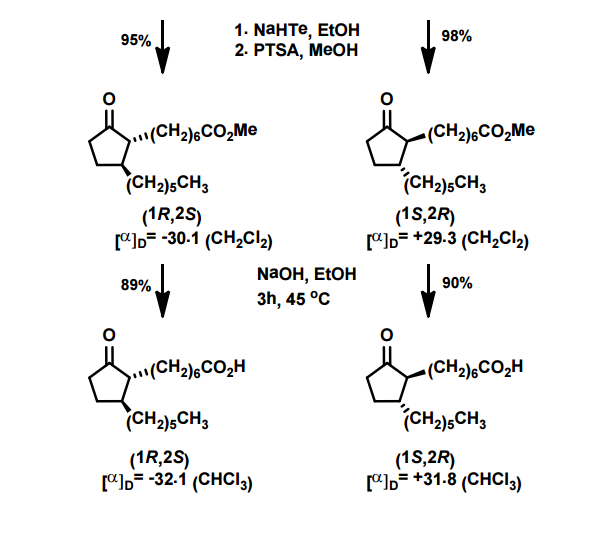
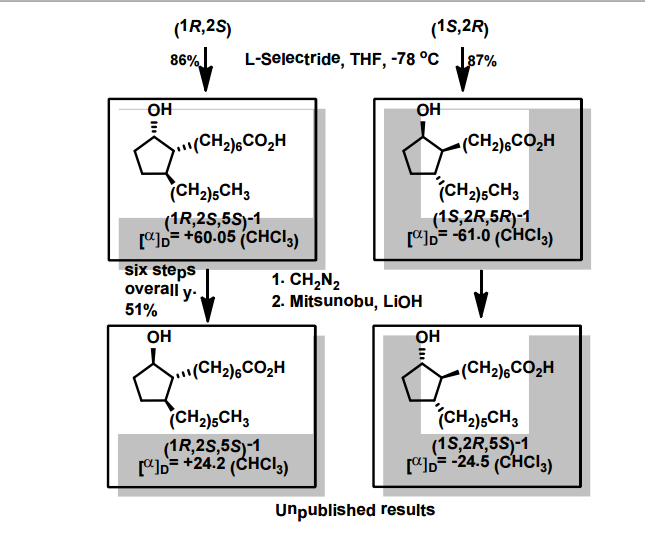
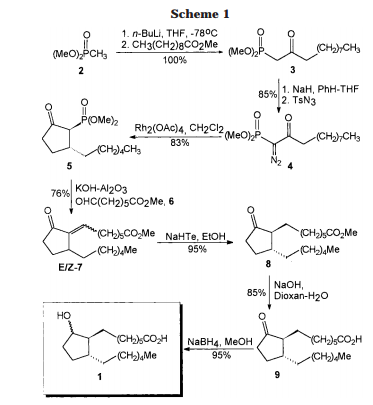
A total synthesis of racemic rosaprostol, an untiulcer drug, has been achieved in seven synthetic steps and in 42% overall yield starting from dimethyl methanephosphonate. The key steps include intramolecular carbenoid cyclization of dimethyl 1-diazo-2-oxoundecanephosphonate 4 leading to 2-dimethoxyphosphoryl-3-hexylcyclopentanone 5 and the Horner−Wittig reaction of the latter with methyl 5-formylpentanecarboxylate 6 employed for the introduction of the methoxycarbonylhexyl moiety at C(2) of the cyclopentanone ring
1 (0.076 g, 95%) as a mixture of trans-trans and trans-cis isomers: Rf ) 0.18 and 0.23 (petroleum ether/Et2O/ AcOH 8:8:0.1);
1H NMR δ 4.19-4.10 (m, 1H), 3.92-3.80 (m, 1H), 2.18 (t, J ) 7.3, 4H), 2.10-1.96 (m, 1H), 1.82-0.85 (m, 51H), 0.93 (t, J ) 6.6, 6H);
13C NMR δ 180.13, 79.93, 75.13, 55.09, 52.71, 45.71, 43.02, 37.15, 36.33, 35.23, 34.95, 34.71, 34.61, 33.03, 30.86, 30.77, 30.69, 30.48, 30.12, 30.03, 29.53, 29.48, 29.21, 28.79, 28.67, 25.68, 23.81, 15.06;
HRMS (CI) (M + H - H2O)+ calcd for C18H33O2 281.2480, obsd 281.2476.
References: Prostaglandin analog. Prepn, hypolipemic, platelet aggregation inhibitory activity: U. Valcavi, DE 2535343,eidem, US 4073938 (1976, 1978 both to Ist. Biochim. Ital.).
Alternate process: V. Marotta, G. Zabban, EP 155392 (1985 to Ist. Biochim. Ital.).
Gastric antisecretory, cytoprotective activity: U. Valcavi et al., Arzneim.-Forsch. 32, 657 (1982).
Effect on mucus and gastrin secretion in duodenal ulcer: D. Foschi et al., Prostaglandins Leukotrienes Med. 15, 147 (1984). Comparison with cimetidine, q.v.: eidem, Drugs Exp. Clin. Res. 10, 427 (1984).
Clinical evaluation in treatment of ulcers: G. P. Tincani et al.,Minerva Med. 78, 847 (1987).
////////////