Bempedoic Acid
ETC-1002, ESP-55016
CAS 738606-46-7
- C19H36O5
- MW 344.486 Da
8-Hydroxy-2.2.14,14-tetramethylpentadecanedioic acid
ATP Citrate Lyase Inhibitor and AMP-activated Protein kinase (AMPK) activator
Indication: Hypercholesterolemia
Development Stage: Phase II
Developer: Esperion Therapeutics
Esperion Therapeutics was founded in April 2008 by former executives of, and investors in, the original Esperion Therapeutics which was founded in July 1998 and was bought by Pfizer for $ 1.3 billion in 2004 and then spun out in 2008. ETC-1002 was first discovered at the original Esperion, and Esperion subsequently acquired the rights to it from Pfizer in 2008. Esperion own the exclusive worldwide rights to ETC-1002.
Bempedoic Acid ( ETC-1002) has a UNIQUE Dual mechanism of Action That has the Potential to Regulate Both lipid and Carbohydrate Metabolism. ETC-1002 Appears to Work by inhibitin ATP citrate lyase (ACL), a Key Enzyme in the Cholesterol biosynthetic pathway, and activating a Complementary Enzyme, 5′-adenosine monophosphate-activated Protein kinase (AMPK). Both Enzymes are Known to Play Significant roles in the synthesis of Cholesterol and glucose in the liver. By inhibitin Cholesterol synthesis in the liver, Causes ETC-1002 the liver to take up LDL particles from the blood, which reduces LDL-C levels.
WO 2004067489


6.13
7-Bromo-2,2-dimethylheptanoic acid ethyl ester
 7-Bromo-2,2-dimethylheptanoic acid ethyl ester
7-Bromo-2,2-dimethylheptanoic acid ethyl esterUnder argon atmosphere and cooling with an ice-bath, a solution of lithium diisopropylamide in THF (1.7 L, 2.0 M, 3.4 mol) was slowly dropped into a solution of 1 ,5- dibromopentane (950 g, 4.0 mol) and ethyl isobutyrate (396 g, 3.4 mol) in THF (5 L) while keeping the temperature below +5 DC. The reaction mixture was stiπed at room temperature for 20 h and quenched by slow addition of saturated ammonium chloride solution (3 L). The resulting solution was divided into three 4-L portions. Each portion was diluted with saturated ammonium chloride solution (5 L) and extracted with ethyl acetate (2 ‘ 2 L). Each 4-L portion of ethyl acetate was washed with saturated sodium chloride solution (2 L), 1 N hydrochloric acid (2 L), saturated sodium chloride solution (2 L), saturated sodium bicarbonate solution (2 L), and saturated sodium chloride solution (2 L). The three separate ethyl acetate layers were combined into a single 12-L portion, dried over magnesium sulfate, and concenfrated in vacuo to give the crude material (1.7 L) which was purified by vacuum distillation. Two fractions were obtained: the first boiling at 88 – 104 °C / 0.6 ton (184.2 g), the second at 105 – 120 °C / 1.4 ton (409.6 g) for atotal yield of 60 %. 1H NMR (300 MHz, CDC13/TMS): δ (ppm): 4.11 (q, 2 H, J = 7.2 Hz), 3.39 (t, 2 H, J = 6.8 Hz), 1.85 (m, 2 H), 1.56 – 1.35 (m, 4 H), 1.24 (t, 3 H, J = 7.2 Hz), 1.31 – 1.19 (m, 2 H), 1.16 (s, 6 H). 13C NMR (75 MHz, CDCI3/TMS): δ (ppm): 177.9, 60.2, 42.1, 40.5, 33.8, 32.6, 28.6, 25.2, 24.2, 14.3. HRMS (El, pos): Calcd. for CπH22Brθ2 (MH+): 265.0803, found: 265.0810.
6.18
2,2,14.14-Tetramethyl-8-oxo-pentadecanedioic acid diethyl ester
 8-isocyano-2,2,14,14-teframethyl-8-(toluene-4-sulfonyl)-pentadecanedioic acid diethyl ester
8-isocyano-2,2,14,14-teframethyl-8-(toluene-4-sulfonyl)-pentadecanedioic acid diethyl ester 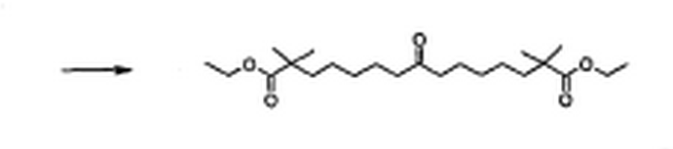 2,2,14,14-tetramethyl-8-oxo-pentadecanedioic acid diethyl ester
2,2,14,14-tetramethyl-8-oxo-pentadecanedioic acid diethyl ester Under Ar atmosphere, to a solution of 7-bromo-2,2-dimethylheptanoic acid ethyl ester (26.50 g, 100 mmol), tetra-n-butylammonium iodide (3.69 g, 10 mmol) and p- toluenesulfonyl methyl isocyanide (9.80 g, 50 mmol) in anhydrous DMSO (300 mL) was added sodium hydride (4.80 g, 20.5 mmol, 60 % dispersion in mineral oil) at 5 – 10 oC. The reaction mixture was stiπed at room temperature for 20 h and quenched with ice-water (300 mL). The product was extracted with dichloromethane (3 D 100 mL). The combined organic layers were washed with water (200 mL), half-saturated NaCl solution (2 ‘ 200 ■– ■ ■• •• ■• .. <i„ ‘ir ι., – ib,
mL), and saturated NaCl solution (200 mL), dried over MgS04, and concentrated in vacuo to get the crude 8-isocyano-2,2,14,14-teframethyl-8-(toluene-4-sulfonyl)-pentadecanedioic acid diethyl ester (36.8 g) as an orange oil, which was used in the next step without purification. To a solution of this crude product (36.8 g) in dichloromethane (450 mL) was added concentrated hydrochloric acid (110 mL) and the mixture was stiπed at room temperature for 1 h. The solution was diluted with water (400 mL) and the aqueous layer was extracted with dichloromethane (200 mL). The combined organic layers were washed with saturated NaHC0 solution (2 x 150 mL) and saturated NaCl solution (150 mL). The organic solution was dried over Na2S04 and concenfrated in vacuo. The residue was subjected to column chromatography (silica gel, hexanes : ethyl acetate = 11 : 1) to give 2,2,14,14-tetramethyl-8-oxo-pentadecanedioic acid diethyl ester (12.20 g, 66 % over two steps) as a colorless oil. lH NMR (300 MHz, CDC13/TMS): δ (ppm): 4.11 (q, 4 H, J – 6.9 Hz), 2.37 (t, 4 H, J – 7.5 Hz), 1.58 – 1.47 (m, 8 H), 1.35 – 1.10 (m, 8 H), 1.24 (t, 6 H, J = 7.2 Hz), 1.15 (s, 12 H). 13C NMR (75 MHz, CDC13/TMS): δ (ppm): 211.6, 178.3, 60.5, 43.1, 42.5, 40.9, 30.1, 25.5, 25.1, 24.1, 14.7. HRMS (LSIMS, nba): Calcd. for C23IL3O5 (MH+): 399.3110, found: 399.3129.
6.19
8-Oxo-2,2,14,14-tetramethylpentadecanedioic acid

A solution of KOH (25 g) in water (50 mL) was added to a solution of 2,2,14,14-tetramethyl-8-oxo-pentadecanedioic acid diethyl ester (10.69 g, 155 mmol) in ethanol (400 mL), then heated at reflux for 4 h. After cooling, the solution was evaporated to a volume of ca. 50 mL and diluted with water (800 mL). The organic impurities were removed by extracting with dichloromethane (2 x 200 mL). The aqueous layer was acidified to pH 2 with concentrated hydrochloric acid (50 mL) and extracted with methyl tert.-butyl ether (MTBE, 3 x 200 mL). The combined organic layers were dried over magnesium sulfate and concenfrated in vacuo to give the crude product (9.51 g) as an oil. Crystallization from hexanes / MTBE (50 mL : 25 mL) afforded 8-oxo-2,2,14,14- teframethylpentadecanedioic acid (6.92 g, 79 %) as waxy, white crystals. M.p.: 83 – 84 °C. 1H NMR (300 MHz, CDCI3/TMS): δ (ppm): 12.03 (s, 2 H), 2.37 (t, 4 H, J = 7.3 Hz), 1.52 – 1.34 (m, 8 H), 1.28 – 1.10 (m, 8 H), 1.06 (s, 12 H). 13C NMR (75 MHz, CDCI3/TMS): δ (ppm): 210.5, 178.8, 41.7, 41.2, 29.1, 25.0, 24.4, 23.1. HRMS (LSIMS, gly): Calcd. for C19H3505 (MH+): 343.2484, found: 343.2485.
6.20
8-Hydroxy-2.2.14,14-tetramethylpentadecanedioic acid
Under nitrogen atmosphere, sodium borohydride (0.06 g, 1.6 mmol) was added to a stiπed solution of 8-oxo-2,2,14,14-tetramethylpentadecanedioic acid (1.18 g, 3.4 mmol) in methanol (50 mL) at 0 °C. The reaction progress was momtored by thin layer chromatography (silica; hexanes : ethyl acetate = 50 : 50). Additional sodium borohydride was added after 1 h (0.48 g, 13 mmol). After 8 h, the reaction mixture was hydrolyzed with water (50 mL) and acidified with concenfrated hydrochloric acid (3 mL) to pH 1. The solution was diluted with water (50 mL) and exfracted with dichloromethane (4 x 25 mL). The combined organic layers were washed with saturated sodium chloride solution (2 x 30 mL), dried over magnesium sulfate, concentrated in vacuo, and dried in high vacuo to give 8-hydroxy-2,2,14,14-tetramethylpentadecanedioic acid (0.7 g, 60 %) as a very viscous oil.
!H NMR (300 MHz, CDC13/TMS): δ (ppm): 7.42 (br. s, 3 H), 3.59 (br. s, 1 H), 1.65 – 1.00 (m, 20 H), 1.18 (s, 12 H). 13C NMR (75 MHz, CDC13/TMS): δ (ppm): 184.5, 71.8, 42.1, 40.5, 37.0, 29.8, 25.2, 25.1, 24.9, 24.8.
HRMS (FAB): Calcd. for Cι9H3705 (MH+): 345.2635, found: 345.2646. HPLC: 83.8 % purity.
………………..
PAPER
Journal of Medicinal Chemistry, 47 (24), 6082-6099;. 2004
http://pubs.acs.org/doi/abs/10.1021/jm040006p
Keto-substituted hydrocarbons with
11−19 methylene and bis-terminal hydroxyl and carboxyl groups have been
synthesized and evaluated in both in vivo and in vitro assays for their
potential to favorably alter lipid disorders including metabolic
syndrome. Compounds were assessed for their effects on the de novo
incorporation of radiolabeled acetate into lipids in primary cultures of
rat hepatocytes as well as for their effects on lipid and glycemic
variables in obese female Zucker fatty rats [Crl:(ZUC)-faBR] following 1
and 2 weeks of oral administration. The most active compounds were
found to be symmetrical with four to five methylene groups separating
the central ketone functionality and the gem dimethyl or
methyl/aryl substituents. Furthermore, biological activity was found to
be greatest in both in vivo and in vitro assays for the
tetramethyl-substituted keto diacids and diols (e.g., 10c, 10g,14c), and the least active were shown to be the bis(arylmethyl) derivatives (e.g., 10e, 10f,14f). Compound 14c
dose-dependently elevated HDL-cholesterol, reduced triglycerides, and
reduced NEFA, with a minimum effective dose of 30 mg/kg/day. Compound 10g
dose-dependently modified non-HDL-cholesterol, triglycerides, and
nonesterified fatty acids, with a minimum effective dose of 10
mg/kg/day. At this dose, compound 10g elevated HDL-cholesterol
levels 2−3 times higher than pretreatment levels, and a dose-dependent
reduction of fasting insulin and glucose levels was observed.
ONLY KETO COMPD DESCRIBED
2,2,14,14-Tetramethyl-8-oxopentadecanedioic Acid (10g). According to the procedure given for 10f, 9g
(8.54 g, 21.4 mmol) was saponified with KOH (85%, 4.53 g, 68.6 mmol) in
EtOH (13 mL) and water (5 mL) at reflux for 4 h. The solid product
obtained after usual workup was recrystallized from Et2O/hexanes (50 mL/50 mL), affording 10g (4.16 g, 57%) as colorless needles.Mp: 82−83 °C.
1H NMR (CDCl3): δ 11.53 (br, 2H), 2.39 (t, 4H, J = 7.3), 1.60−1.50 (m, 8 H), 1.30−1.20 (m, 8 H), 1.18 (s, 12 H).
13C NMR (CDCl3): δ 211.7, 185.0, 42.8, 42.3, 40.4, 29.7, 25.1, 24.8, 23.8.
HRMS (LSIMS, gly): calcd for C19H35O5 (MH+) 343.2484, found 343.2444.
HPLC: Alltima C-8 column, 250 × 4.6 mm, 5 μm; 60% acetonitrile/40% 0.05 M KH2PO4, flow rate 1.0 mL/min; RI, tR 6.50 min, 92.6% pure.
Anal. (C19H34O5): C, H.
 A PRECURSOR OF Bempedoic Acid
A PRECURSOR OF Bempedoic AcidPATENTs/PAPERS
WO2005068412,
WO2004067489,
Journal of Medicinal Chemistry, 47 (24), 6082-6099;. 2004
US20040198814
Esperion Therapeutics
Ringing the bell were Roger Newton, Esperion’s founder and chief science officer, and Tim Mayleben, the CEO and president.
Esperion raised about $73 million in its offering on June 26. It hopes to use the funds to conduct two Phase 3 U.S. Food and Drug Administration trials next year on a cholesterol-lowering drug with the working name of ETC-1002.
ETC-1002 has shown good results in preliminary human trials in lowering LDL, the so-called bad cholesterol, in patients who are either intolerant or resistant to such statin drugs as Lipitor and Torvast. More results are expected this summer.
This was the second IPO for a drug company called Esperion. The first Esperion was founded in 1998 to create a drug to raise HDL, the so-called good cholesterol. It went public in 2000 and was sold to Pfizer Inc. for $1.3 billion in 2004.
In 2008, as part of closing its Michigan operations, Pfizer sold the name and rights to some small molecules back to Newton.

Esperion President and CEO Tim Mayleben (left)
and Chief Science Officer Roger Newton in the company’s labs at the
Michigan Life Science and Innovation Center.Mayleben previously told
AnnArbor.com that the drug being developed by the company, which is
housed at the Michigan Life Science and Innovation Center
in Plymouth Township, is undergoing the second round of “phase two”
clinical tests. Most drugs go through three phases of testing before the
results are submitted to the Food and Drug Administration. Mayleben
said he does not expect the company to submit ETC-1002 to the FDA for
approval for at least another three years.,Newton, a co-inventor of
Lipitor and founder of the first Esperion, raised more than $22 million
to buy the intellectual property from the original company back from
Pfizer when the company closed its Ann Arbor offices in 2007…….http://www.annarbor.com/business-review/ann-arbor-pharmaceutical-company-esperion-therapeutics-to-ring-nasdaq-opening-bell-wednesday/
……………………
Michigan Life Science and Innovation Center in Plymouth Township