GSK 525762A; 1260907-17-2; I-BET-762; GSK525762A; UNII-5QIO6SRZ2R; 5QIO6SRZ2R;
CAS1260907-17-2
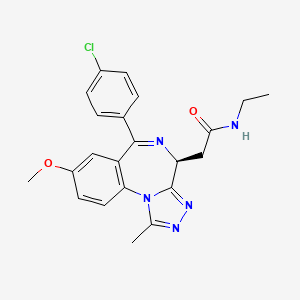
2-[(4S)-6-(4-chlorophenyl)-8-methoxy-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepin-4-yl]-N-ethylacetamide
| Molecular Formula: | C22H22ClN5O2 |
|---|---|
| Molecular Weight: | 423.89538 g/mol |
| Solubility: | Soluble in DMSO (84 mg/ml at 25 °C), ethanol (42 mg/ml at 25 °C, warmed), DMF (~30 mg/ml), ethanol:PBS (pH 7.2, 1:1) (~0.5 mg/ml), and water (<1 mg/ml at 25 °C). |
| Storage: | Store at -20° C |
| Density: | ~1.4 g/cm3 (Predicted) |
| Refractive Index: | n20D 1.67 (Predicted) |
| Optical Activity: | α20D 85º±5º, c = 0.3 in methanol |
| IC50: | BRD2: IC50 = 32.5 nM (human); BRD4: IC50 = 36.1 nM (human); BRD3: IC50 = 42.4 nM (human); PBMC: IC50 = 316.23 nM (human); HepG2: EC5050 = 700 nM (human) |
| pK Values: | pKb: 2.43 (Predicted) |
GSK 525762A, is a BET Bromodomain Inhibitor, which is now in clinical development. BET bromodomains have emerged as promising drug targets for treatment of cancers, inflammatory diseases, and other medical conditions.
Patent
WO-2016050821
Patent applications WO201 1/054553 and WO201 1/054845 (both in the name of GlaxoSmithKline LLC) disclose the compound 2-[(4S)-6-(4-chlorophenyl)-1-methyl-8-(methyloxy)-4/-/-[1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]-/V-ethylacetamide as a BET family bromodomain inhibitor and describes therapeutic uses thereof. The chemical structure of this compound is represented by formula (I):

(I)
Scheme 1



Example 1
Preparation of an acetonitrile solvate of 2-[(4S)-6-(4-chlorophenyl)-1 -methyl-8-(methyloxy)-4H-[1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]-yV-ethylacetamide
Amorphous 2-[(4S)-6-(4-chlorophenyl)-1-methyl-8-(methyloxy)-4H-[1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]-/V-ethylacetamide (prepared for instance as described in WO201 1/054553, 1 wt) was dissolved in acetonitrile (20 vol) upon heating (up to reflux). The solution was then distilled to 10 vol keeping the temp 50 °C - 60 °C by adjusting the vacuum. Nucleation occurred during the final stage of the distillation. The slurry was then held at 60 °C before being cooled to 20 °C and filtered. The cake was then washed with
acetonitrile (2 vol). The cake was dried under vacuum with a nitrogen bleed at approximately 60 °C to provide the titled product.
1H-NMR (500 MHz, DMSO-d6, referenced to TMS = 0.00 ppm, T = 25 C) δ ppm 8.22 (1 H, t, J = 5 Hz), 7.79 (1 H, d, J = 9 Hz), 7.53 (2H, d, J = 9 Hz), 7.49 (2H, d, J = 9 Hz), 7.38 (1 H, dd, J = 3 Hz, 9 Hz), 6.87 (1 H, d, J = 3 Hz), 4.49 (1 H, m), 3.79 (3H, s), 3.25 (1 H, m), 3.20-3.06 (3H, several m), 2.54 (3H, s), 2.08 (3H, s), 1 .07 (3H, t, J = 7 Hz).
Example 2
Preparation of a benzene sulphonic acid salt of 2-[(4S)-6-(4-chlorophenyl)-1 -methyl-8-(methyloxy)-4H-[1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]-A/-ethylacetamide in crystalline solid state form
Preparation 1
The acetonitrile solvate of 2-[(4S)-6-(4-chlorophenyl)-1-methyl-8-(methyloxy)-4/-/-[1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]-/V-ethylacetamide (for a preparation see Example 1 , 2.58 g) was slurried in acetonitrile (7 mL) and 2-methyltetrahydrofuran (7 mL). Benzenesulfonic acid (1.17 g) was dissolved in acetonitrile (7 mL). The resulting solution was charged to the slurry of 2-[(4S)-6-(4-chlorophenyl)-1-methyl-8-(methyloxy)-4/-/-[1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]-/V-ethylacetamide acetonitrile solvate in acetonitrile and 2-methyltetrahydrofuran. An additional rinse of acetonitrile (1.4 mL) and 2-methyltetrahydrufran (0.7 mL) was added to the slurry. The slurry was then warmed to 60 °C to dissolve. 2-methyltetrahydrofuran (50 mL) was then added over 30 minutes. Crystals formed during this addition. The resulting suspension was then cooled to 5 °C at a controlled, linear rate of 0.5 °C/minute. The slurry was aged for 1 hour. The crystalline product was then isolated by filtration and rinsed with a 5 to 1 mixture of 2-methyltetrahydrofuran and acetonitrile (15 mL). The product was then dried in a vacuum oven at 55 °C overnight.
Preparation 2
The acetonitrile solvate of 2-[(4S)-6-(4-chlorophenyl)-1 -methyl-8-(methyloxy)-4/-/-[1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]-/V-ethylacetamide (prepared for example in a process such as Example 1 above, 1 wt) was dissolved in 9 vol 2-methyltetrahydrofuran at 65 °C. Once cooled to 20°C the solution was filtered into the crystallization vessel. The dissolution vessel and inline filter were rinsed with 1 vol 2-methyltetrahydrofuran. The solution was then heated to 45 °C.
1 .05 eq of benzene sulphonic acid was dissolved in 1 volume of filtered acetonitrile. 10% of this solution was added to a reactor to which 0.05 wt% of a benzene sulphonic acid
salt of 2-[(4S)-6-(4-chlorophenyl)-1-methyl-8-(methyloxy)-4H-[1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]-/V-ethylacetamide micronized seed (prepared for example as in Preparation 1 above) slurry was charged. The remaining benzene sulphonic acid solution was charged at a steady rate over 2 hours, maintaining the reactor at 45 °C.
The slurry was cooled to 0 °C at no greater than 0.2 °C/minute. The slurry was filtered.
The crystallizer was charged with the first wash, 3 vol of filtered 2-methyltetrahydrofuran, which was cooled to <10 °C while stirring in the crystallizer, before being used to wash the cake. The crystallizer was charged with the second wash, 3 vol of filtered 2-methyltetrahydrofuran, which was cooled to <10 °C while stirring in the crystallizer, before being used to wash the cake. The crystallizer was charged with the third wash, 4 vol of filtered 2-MeTHF, which was cooled to <10 °C while stirring in the crystallizer, before being used to wash the cake. The cake was blown-down until the solvent being removed was reduced to a trickle. The title compound was then dried in a vacuum oven at 50 °C until the loss on drying (LOD) indicates <0.2% wt. loss (LOD method: 10 min at 120 °C). The product was then delumped using a comil.
Example 3
Characterisation of a benzene sulphonic acid salt of 2-[(4S)-6-(4-chlorophenyl)-1 -methyl-8-(methyloxy)-4H-[1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]-yV-ethyl acetamide in crystalline solid state form
XRPD
The X-ray powder diffraction (XRPD) data were acquired on a PANalytical X'Pert Pro powder diffractometer, model PW3050/60, using an X'Celerator detector. The acquisition conditions were: radiation: Cu Ka, generator tension: 45 kV, generator current: 40 mA, step size: 0.017 °2Θ, time per step: 500 seconds, divergence slit type: fixed, divergence slit size: 0.4354 °, measurement temperature: 20-25 °C, goniometer radius: 240 mm. The sample was prepared by packing sample in a 0.9 mm capillary. Peak positions were obtained using PANalytical X'Pert Highscore Plus software. The margin of error is approximately ± 0.1° 2Θ for each of the peak assignments.
The X-ray powder diffraction (XRPD) pattern is shown in Figure 1 and shows characteristic peaks, expressed in degrees 2Θ, at 5.5, 7.4, 9.1 , 10.0, 10.4, 13.3, 13.6, 14.9, 18.7, 20.4, 20.9, 22.8 and 23.1 ° ( ± 0.1 °).
13C Solid State NMR (SSNMR)
A 13C SSNMR spectrum was obtained at 273K on a spectrometer operating at a frequency of 100.56 MHz for 13C observation using a cross-polarization pulse sequence with a Bruker 4-mm triple resonance magic-angle spinning probe at a rotor frequency of 8 kHz. The margin of error is ± 0.2 ppm for each of the peak assignments.
The 13C SSNMR spectrum is shown in Figure 2 and comprises chemical shifts (ppm) at 169.6, 167.5 165.6, 160.1 , 159.4, 157.1 , 155.9, 154.3, 152.4, 146.9, 145.8, 140.0, 137.9, 135.9, 133,4, 132.0, 130.6, 129.9, 128.3, 127.1 , 125.6, 123.5, 120.6, 1 19.1 , 1 14.1 , 1 13.7, 58.0, 53.6, 53.1 , 40.7, 37.0, 34.9, 15.8, 14.7, and 12.0 ( ±0.2 ppm).
PATENT
WO2011054553
http://www.google.com/patents/WO2011054553A1?cl=en
formula (I) which is 2-[(4S)-6-(4- Chlorophenyl)-1-methyl-8-(methyloxy)-4H-[1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]-/V- ethylacetamide
(I)
or a salt thereof.
It will be appreciated that the present invention covers compounds of formula (I) as the free base and as salts thereof, for example as a pharmaceutically acceptable salt thereof.
In one embodiment there is provided a compound which is 2-[(4S)-6-(4-Chlorophenyl)-1- methyl-8-(methyloxy)-4H-[1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]-/V-ethylacetamide.
Because of their potential use in medicine, salts of the compounds of formula (I) are desirably pharmaceutically acceptable. In another embodiment there is provided a compound which is 2-[(4S)-6-(4-Chlorophenyl)-1-methyl-8-(methyloxy)-4H- [1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]-/V-ethylacetamide or a pharmaceutically acceptable salt thereof.
The compound of formula (I) may be prepared according to reaction scheme 1 by reaction of a compound of formula (II) with EtNH2 in the presence of HATU or HBTU and DIEA at room temperature. Alternatively compounds of formula (I) may be prepared by reacting the compound of formula (II) with oxalyl chloride followed by addition of EtNH2 in the presence of triethylamine.
Scheme 1
The compound of formula (II) may be prepared according to reaction Scheme 2. Suitable reaction conditions comprise reacting a compound of formula (III) with alkaline hydroxide preferably sodium hydroxide or lithium hydroxide.
Scheme 2
wherein R represents C-|.galkyl such as methyl.
Compounds of formula (III), may be prepared according to reaction scheme 3 by reacting compounds of formula (IV) with AcOH. Scheme 3
Compounds of formula (IV) may be prepared according to reaction scheme 4 by reacting compounds of formula (VI) with hydrazine below 15 °C followed by reaction of the resulting hydrazone (V) with MeCOCI at 0°C. Generally hydrazone (V) is used without further purification and is reacted with MeCOCI at , for example 0 °C.
Scheme 4
(IV) Compounds of formula (VI) in which R is Ci-6alkyl (such as methyl) may be prepared according to reaction scheme 5 from compounds of formula (VII) by treatment with Lawesson's reagent or P4Si0. Suitable reaction conditions comprise reacting compounds of formula (VIII) with P4Si0 in 1 ,2-dichloroethane at, for example 70 °C.
Scheme 5
Compounds of formula (VII) may be prepared according to reaction scheme 6, by reacting compounds of formula (IX) with an organic base such as triethylamine followed by reaction of the resulting amine (VIII) with acetic acid. Generally, amine (VIII) is used without further purification and is reacted with AcOH at, for example 60 °C.
Scheme 6
Compounds of formula (IX) may be prepared according to reaction scheme 7, by reacting compounds of formula (XI) with the acylchloride (X) derived from protected aspartic acid. Scheme 7
Compounds of formula (XI) may be prepared according to procedures described in Synthesis 1980, 677-688. Acyl chlorides of formula (X) may be prepared according to procedures described in J. Org. Chem., 1990, 55, 3068-3074 and J. Chem. Soc. Perkin Trans. 1 , 2001 , 1673-1695.
Alternatively the compound of formula (I) may be prepared according to reaction scheme 8.
wherein R represents C-|_4alkyl such as methyl.
The compound of formula (IIIA) may be prepared according to reaction scheme 9 by reacting compounds of formula (IVA) with EtNH2 in the presence of HATU and DIEA at, for example room temperature.
Scheme 9
The compound of formula (IVA) may be prepared according to reaction scheme 10. Suitable reaction conditions comprise reacting compounds of formula (VI) with alkaline hydroxide such as sodium hydroxide. Scheme 10
Example 1 : 2-[(4S)-6-(4-Chlorophenyl)-1 -methyl-8-(methyloxy)-4H-[1 ,2,4]triazolo[4,3-
To a solution of [(4S)-6-(4-Chlorophenyl)-1-methyl-8-(methyloxy)-4H-[1 !2!4]triazolo[4,3- a][1 ,4]benzodiazepin-4-yl]acetic acid (for a preparation see Intermediate 1 )(16.0 g, 40 mmol) in THF at RT was added DIEA (14 mL, 80 mmol) followed by HATU (30.4 g, 80 mmol). The reaction mixture was stirred for 3h at this temperature and ethylamine (40 mL, 2M in THF, 80 mmol) was added. The mixture was stirred for 48h before being concentrated under reduced pressure. The crude material was suspended in water and extracted with DCM. The organic layer was dried over Na2S04, filtered and concentrated in vacuo. The crude solid was purified by chromatography on Si02 (DCM/MeOH 95/5) and the resulting solid recrystallised in MeCN. The solid was then dissolved in DCM and precipited with /'-Pr20 to give the title compound (8 g, 47% yield) as a white solid.
Rf = 0.48 (DCM/MeOH : 90/10). Mp >140 °C (becomes gummy). 1H NMR (300 MHz, CDCI3) 7.53-7.47 (m, 2H), 7.39 (d, J = 8.9 Hz, 1 H), 7.37-7.31 (m, 2H), 7.20 (dd, J = 2.9 and 8.9 Hz, 1 H), 6.86 (d, J = 2.9 Hz, 1 H), 6.40 (m, 1 H), 4.62 (m, 1 H), 3.80 (s, 3H), 3.51 (dd, J = 7.3 and 14.1 Hz, 1 H), 3.46-3.21 (m, 3H), 2.62 (s, 3H), 1.19 (t, J = 7.3 Hz, 3H). LC/MS : m/z 424 [M(35CI)+H]+, Rt 2.33 min.
Intermediate 1 : [(4S)-6-(4-Chlorophenyl)-1 -methyl-8-(methyloxy)-4H-
[1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]acetic acid
To a solution of methyl [(4S)-6-(4-chlorophenyl)-1 -methyl-8-(methyloxy)-4H- [1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]acetate (for a preparation see Intermediate 2)(28 g, 68 mmol) in THF (450 mL) at RT was added 1 N NaOH (136 mL, 136 mmol). The reaction mixture was stirred at this temperature for 5h before being cooled down and quenched with 1 N HCI (136 mL). THF was removed under reduced pressure and the aqueous layer was extracted with DCM. The combined organic layers were dried over Na2S04, filtered and concentrated under reduced pressure. The crude solid was recrystallised in CH3CN to give the title compound (23.9 g, 89% yield) as a pale yellow powder. 1H NMR (300 MHz, CDCI3) δ 7.55-7.48 (m, 2H), 7.41 (d, J = 8.9 Hz, 1 H), 7.38- 7.31 (m, 2H), 7.22 (dd, J = 2.9 and 8.9 Hz, 1 H), 6.90 (d, J = 2.9 Hz, 1 H), 4.59 (dd, J = 6.9 and 6.9 Hz, 1 H), 3.81 (s, 3H), 3.70 (dd, J = 6.9 and 25.7 Hz, 1 H), 3.61 (dd, J = 6.9 and 25.7 Hz, 1 H), 2.63 (s, 3H). LC/MS: m/z 397 [M(35CI)+H]+, Rt 2.1 1 min.
Intermediate 2: Methyl [(4S)-6-(4-chlorophenyl)-1 -methyl-8-(methyloxy)-4H- [1 ,2,4]triazolo[4,3-a][1 ,4]benz
To crude methyl [(3S)-2-[(1 Z)-2-acetylhydrazino]-5-(4-chlorophenyl)-7-(methyloxy)-3H- 1 ,4-benzodiazepin-3-yl]acetate (for a preparation see Intermediate 3) (34 g, 79 mmol) was suspended in THF (200 mL) and AcOH (200 mL) was added at RT. The reaction mixture was stirred at this temperature overnight before being concentrated to dryness. The residue was suspended in saturated NaHC03 and extracted with DCM. The organic layer was dried over Na2S04, filtered and concentrated in vacuo. The crude solid was purified by chromatography on Si02 (DCM/MeOH : 90/10) to give the title compound (28 g, 86% yield) as a yellow powder.
1H NMR (300 MHz, CDCI3) δ 7.54-7.47 (m, 2H), 7.40 (d, J = 8.8 Hz, 1 H), 7.37-7.31 (m, 2H), 7.22 (dd, J = 2.8 and 8.8 Hz, 1 H), 6.89 (d, J = 2.8 Hz, 1 H), 4.61 (dd, J = 6.4 and 7.8 Hz, 1 H), 3.82 (s, 3H), 3.78 (s, 3H), 3.66 (dd, J = 7.8 and 16.9 Hz, 1 H), 3.60 (dd, J = 6.4 and 16.9 Hz, 1 H), 2.62 (s, 3H). LC/MS m/z 41 1 [M(35CI)+H]+, Rt 2.88 min. Intermediate 3: Methyl [(3S)-2-[2-acetylhydrazino]-5-(4-chlorophenyl)-7-(methyloxy)- 3H-1 ,4-benzodiazepin-3-yl]acetate
To a suspension of methyl [(3S)-5-(4-chlorophenyl)-7-(methyloxy)-2-thioxo-2,3-dihydro- 1 H-1 ,4-benzodiazepin-3-yl]acetate (for a preparation see Intermediate 4)(30.2 g, 77.7 mmol) in THF (800 mL) at 0°C was added hydrazine monohydrate (1 1 .3 ml_, 233 mmol) dropwise. The reaction mixture was stirred for 4h between 0°C and 15°C before being cooled at 0°C. Et3N (32.4 mL, 230 mmol) was then added slowly and AcCI (16.3 mL, 230 mmol) was added dropwise. The mixture was allowed to warm to RT and stir for 1 h then quenched with water and concentrated under reduced pressure. The resulting aqueous layer was then extracted with DCM and the organic layer was dried over Na2S04, filtered and concentrated in vacuo to give the crude title compound (34 g, 100% yield) which was used without further purification. LC/MS: m/z 429 [M(35CI)+H]+, Rt 2.83 min. Intermediate 4: Methyl [(3S)-5-(4-chlorophenyl)-7-(methyloxy)-2-thioxo-2,3-dihydro- 1H-1 ,4-benzodiazepin-3-yl]acetate
A suspension of P4Si0 (85.8 g, 190 mmol) and Na2C03 (20.5 g, 190 mmol) in 1 ,2-DCE (1.5 L) at RT was stirred for 1 h before methyl [(3S)-5-(4-chlorophenyl)-7-(methyloxy)-2- oxo-2,3-dihydro-1 H-1 ,4-benzodiazepin-3-yl]acetate (for a preparation see Intermediate 5) (40 g, 107 mmol) was added. The resulting mixture was stirred at 65°C for 4 h before being cooled and filtered. The solid was washed with DCM and the filtrate washed with sat. NaHC03. The organic layer was dried over Na2S04, filtered and concentrated under reduced pressure. The title compound was precipitated from a DCM//'-Pr20 mixture and filtered. The filtrate was then concentrated and purified by flash chromatography (DCM/MeOH : 98/2) to afford another batch of product. The title compound was obtained combining the two fractions (30.2 g, 73%) as a yellow powder. LC/MS: m/z 389
[M(35CI)+H]+, Rt 3.29 min.
Intermediate 5: Methyl [(3S)-5-(4-chlorophenyl)-7-(methyloxy)-2-oxo-2,3-dihydro-1H- 1 ,4-benzodiazepin-3-yl]acetat
To a solution of the crude methyl /V1-[2-[(4-chlorophenyl)carbonyl]-4-(methyloxy)phenyl]- /V2-{[(9H-fluoren-9-ylmethyl)oxy]carbonyl}-L-a-asparaginate (for a preparation see Intermediate 6) (assumed 0.2 mol) in DCM (500 mL) was added Et3N (500 mL, 3.65 mol) and the resulting mixture was refluxed for 24h before being concentrated. The resulting crude amine was dissolved in 1 ,2-DCE (1.5 L) and AcOH (104 mL, 1.8 mol) was added carefully. The reaction mixture was then stirred at 60°C for 2h before being concentrated in vacuo and dissolved in DCM. The organic layer was washed with 1 N HCI and the aqueous layer was extracted with DCM (x3). The combined organic layers were washed twice with water, and brine, dried over Na2S04, filtered and concentrated under reduced pressure. The crude solid was recrystallised in MeCN leading to the title compound (51 g) as a pale yellow solid. The filtrate could be concentrated and recrystallised in MeCN to give another 10 g of Intermediate 9 (total: 61 g, 69% yield based on recovered
Intermediate 12). Rf = 0.34 (DCM/MeOH : 95/5). LC/MS m/z 373 [M(35CI)+H]+, Rt 2.76 min.
Intermediate 6: Methyl W^2-[(4 :hlorophenyl)carbonyl]-4-(methyloxy)phenyl] V2- {[(9H-fluoren-9-ylmethyl)oxy]carbonyl}-L-a-asparaginate
A mixture of Methyl /V-{[(9H-fluoren-9-ylmethyl)oxy]carbonyl}-L-a-aspartyl chloride (prepared from J. Org. C em. 1990, 55, 3068-3074 and J. C em. Soc. Perkin Trans. 1 2001 , 1673-1695) (221 g, 0.57 mol) and [2-amino-5-(methyloxy)phenyl](4- chlorophenyl)methanone (for a preparation see Intermediate 7) (133 g, 0.5 mol) in CHCI3 (410 mL) was stirred at 60°C for 1.5h before being cooled and concentrated under reduced pressure and used without further purification. LC/MS: m/z 613 [M(35CI)+H]+, Rt = 3.89 min. Intermediate 7: [2-amino-5-(methyloxy)phenyl](4-chlorophenyl)methanone
To a solution of 2-methyl-6-(methyloxy)-4H-3,1-benzoxazin-4-one (for a preparation see Intermediate 8)(40.0 g, 0.21 mol) in a toluene (560 ml_)/ether (200 mL) mixture at 0°C was added dropwise a solution of 4-chlorophenylmagnesium bromide (170 mL, 1 M in Et20, 0.17 mol). The reaction mixture was allowed to warm to RT and stirred for 1 h before being quenched with 1 N HCI. The aqueous layer was extracted with EtOAc (3 x) and the combined organics were washed with brine, dried over Na2S04, filtered and concentrated under reduced pressure. The crude compound was then dissolved in EtOH (400 mL) and 6N HCI (160 mL) was added. The reaction mixture was refluxed for 2 h before being concentrated under reduced pressure. The resulting solid was filtered and washed twice with ether before being suspended in EtOAc and neutralised with 1 N NaOH. The aqueous layer was extracted with EtOAc (3 x) and the combined organics were washed with brine, dried over Na2S04, filtered and concentrated under reduced pressure. The title compound was obtained as a yellow solid (39 g, 88 % yield) which was used without further purification. Intermediate 8 : 2-methyl-6-(methyloxy)-4H-3,1 -benzoxazin-4-one
A solution of 5-methoxyanthranilic acid (7.8 g, 46.5 mmol) was refluxed in acetic anhydride (60 mL) for 2h15 before being cooled and concentrated under reduced pressure. The crude residue was then concentrated twice in the presence of toluene before being filtered and washed with ether to yield to the title compound (6.8 g, 77% yield) as a beige solid; LC/MS: m/z 192 [M+H]+, Rt 1.69 min.
Preparation of reference compound for use in biological assays
Experimental details of LC-MS methods A and B as referred to herein are as follows:
LC/MS (Method A) was conducted on a Supelcosil LCABZ+PLUS column (3μηΊ, 3.3cm x 4.6mm ID) eluting with 0.1 % HCO2H and 0.01 M ammonium acetate in water (solvent A), and 95% acetonitrile and 0.05% HCO2H in water (solvent B), using the following elution gradient 0-0.7 minutes 0%B, 0.7-4.2 minutes 0→100%B, 4.2-5.3 minutes 100%B, 5.3-5.5 minutes 100→0%B at a flow rate of 3 mL/minute. The mass spectra (MS) were recorded on a Fisons VG Platform mass spectrometer using electrospray positive ionisation [(ES+ve to give [M+H]+ and [M+NH4]+ molecular ions] or electrospray negative ionisation
[(ES-ve to give [M-H]- molecular ion] modes. Analytical data from this apparatus are given with the following format : [M+H]+ or [M-H]-.
LC/MS (Method B) was conducted on an Sunfire C18 column (30mm x 4.6mm i.d. 3.5μηι packing diameter) at 30 degrees centigrade, eluting with 0.1 % v/v solution of Trifluoroacetic Acid in Water (Solvent A) and 0.1 % v/v solution of Trifluoroacetic Acid in Acetonitrile (Solvent B) using the following elution gradient 0-0.1 min 3%B, 0.1- 4.2min 3 - 100% B, 4.2-4.8min 100% B, 4.8-4.9min 100-3%B, 4.9 - 5.0min 3% B at a flow rate of 3ml/min. The UV detection was an averaged signal from wavelength of 210nm to 350nm and mass spectra were recorded on a mass spectrometer using positive electrospray ionization. Ionisation data was rounded to the nearest integer. LC/HRMS: Analytical HPLC was conducted on a Uptisphere-hsc column (3μηι 33 x 3 mm id) eluting with 0.01 M ammonium acetate in water (solvent A) and 100% acetonitrile (solvent B), using the following elution gradient 0-0.5 minutes 5% B, 0.5-3.75 minutes 5→100% B, 3.75-4.5 100% B, 4.5-5 100→5% B, 5-5.5 5% B at a flow rate of 1 .3 mL/minute. The mass spectra (MS) were recorded on a micromass LCT mass spectrometer using electrospray positive ionisation [ES+ve to give MH+ molecular ions] or electrospray negative ionisation [ES-ve to give (M-H)- molecular ions] modes.
TLC (thin layer chromatography) refers to the use of TLC plates sold by Merck coated with silica gel 60 F254.
Silica chromatography techniques include either automated (Flashmaster or Biotage SP4) techniques or manual chromatography on pre-packed cartridges (SPE) or manually- packed flash columns.
Reference compound A : 2-meth -6-(methyloxy)-4H-3,1 -benzoxazin-4-one
A solution of 5-methoxyanthranilic acid (Lancaster) (41.8 g, 0.25 mol) was refluxed in acetic anhydride (230 mL) for 3.5 h before being concentrated under reduced pressure. The crude compound was then concentrated twice in the presence of toluene before being filtered and washed twice with ether to yield to the title compound (33.7 g, 71 % yield) as a brown solid; LC/MS (Method A): m/z 192 [M+H]+, Rt 1.69 min.
Reference compound B: [2-amino- -(methyloxy)phenyl](4-chlorophenyl)methanone
To a solution of 2-methyl-6-(methyloxy)-4H-3,1-benzoxazin-4-one (for a preparation see Reference compound A) (40.0 g, 0.21 mol) in a toluene/ether (2/1 ) mixture (760 mL) at 0°C was added dropwise a solution of 4-chlorophenylmagnesium bromide (170 mL, 1 M in Et20, 0.17 mol). The reaction mixture was allowed to warm to room temperature and stirred for 1 h before being quenched with 1 N HCI (200 mL). The aqueous layer was extracted with EtOAc (3 x 150 mL) and the combined organics were washed with brine (100 mL), dried over Na2S04, filtered and concentrated under reduced pressure. The crude compound was then dissolved in EtOH (400 mL) and 6N HCI (160 mL) was added. The reaction mixture was refluxed for 2 h before being concentrated to one-third in volume. The resulting solid was filtered and washed twice with ether before being suspended in EtOAc and neutralised with 1 N NaOH. The aqueous layer was extracted with EtOAc (3 x 150 mL) and the combined organics were washed with brine (150 mL), dried over Na2S04, filtered and concentrated under reduced pressure. The title compound was obtained as a yellow solid (39 g, 88 % yield); LC/MS (Method A): m/z 262 [M+H]+, Rt 2.57 min.
Reference Compound C: Methyl /^-^-[(^chlorophenyljcarbonyl]^- (methyloxy)phenyl]-yV2-{[(9H-fluoren-9-ylmethyl)oxy]carbonyl}-L-a-asparaginate
Methyl /V-{[(9H-fluoren-9-ylmethyl)oxy]carbonyl}-L-a-aspartyl chloride {Int. J. Peptide Protein Res. 1992, 40, 13-18) (93 g, 0.24 mol) was dissolved in CHCI3 (270 mL) and [2- amino-5-(methyloxy)phenyl](4-chlorophenyl)methanone (for a preparation see Reference compound B) (53 g, 0.2 mol) was added. The resulting mixture was stirred at 60°C for 1 h before being cooled and concentrated at 60% in volume. Ether was added at 0°C and the resulting precipitate was filtered and discarded. The filtrate was concentrated under reduced pressure and used without further purification.
Reference compound D: Methyl [(3S)-5-(4-chlorophenyl)-7-(methyloxy)-2-oxo-2,3- dihydro-1H-1 ,4-benzodiazepin-3-yl]acetate
To a solution of Methyl N1-[2-[(4-chlorophenyl)carbonyl]-4-(methyloxy)phenyl]-N2-{[(9H- fluoren-9-ylmethyl)oxy]carbonyl}-L-a-asparaginate (for a preparation see Reference compound C) (assumed 0.2 mol) in DCM (500 mL) was added Et3N (500 mL, 3.65 mol) and the resulting mixture was refluxed for 24h before being concentrated. The resulting crude amine was dissolved in 1 ,2-DCE (1.5 L) and AcOH (104 mL, 1.8 mol) was added carefully. The reaction mixture was then stirred at 60°C for 2h before being concentrated in vacuo and dissolved in DCM. The organic layer was washed with 1 N HCI and the aqueous layer was extracted with DCM (x3). The combined organic layers were washed twice with water, and brine, dried over Na2S04, filtered and concentrated under reduced pressure. The crude solid was recrystallised in MeCN leading to the title compound (51 g) as a pale yellow solid. The filtrate could be concentrated and recrystallised in MeCN to give to another 10 g of the desired product Rf = 0.34 (DCM/MeOH : 95/5).
HRMS (M+H)+ calculated for C19H18 35CIN204 373.0955; found 373.0957.
Reference compound E: Methyl [(3S)-5-(4-chlorophenyl)-7-(methyloxy)-2-thioxo-2,3- dihydro-1 H-1 ,4-benzodiazepi -3-yl]acetate
A suspension of P4Si0 (36.1 g, 81.1 mmol) and Na2C03 (8.6 g, 81.1 mmol) in 1 ,2-DCE (700 mL) at room temperature was stirred for 2 h before Methyl [(3S)-5-(4-chlorophenyl)- 7-(methyloxy)-2-oxo-2,3-dihydro-1 H-1 ,4-benzodiazepin-3-yl]acetate (for a preparation see Reference compound D) (16.8 g, 45.1 mmol) was added. The resulting mixture was stirred at 70°C for 2 h before being cooled and filtered. The solid was washed twice with DCM and the filtrate washed with sat. NaHC03 and brine. The organic layer was dried over Na2S04, filtered and concentrated under reduced pressure. The crude product was purified by flash-chromatography on silica gel (DCM/MeOH : 99/1 ) to afford the title compound (17.2 g, 98% yield) as a yellowish solid. LC/MS (Method A): m/z 389 [M(35CI)+H]+, Rt 2.64 min
HRMS (M+H)+ calculated for C19H18 35CIN203S 389.0727; found 389.0714. Reference compound F: Methyl [(3S)-2-[2-acetylhydrazino]-5-(4-chlorophenyl)-7- (methyloxy)-3H-1 ,4-benzodiazepin-3- l]acetate
To a suspension of Methyl [(3S)-5-(4-chlorophenyl)-7-(methyloxy)-2-thioxo-2,3-dihydro- 1 H-1 ,4-benzodiazepin-3-yl]acetate (for a preparation see Reference compound E (9.0 g, 23.2 mmol) in THF (300 mL) at 0°C was added hydrazine monohydrate (3.4 mL, 69.6 mmol) dropwise. The reaction mixture was stirred for 5h between 5°C and 15°C before being cooled at 0°C. Et3N (9.7 mL, 69.6 mmol) was then added slowly and acetyl chloride (7.95 mL, 69.6 mmol) was added dropwise. The mixture was then allowed to warm to room temperature for 16h before being concentrated under reduced pressure. The crude product was dissolved in DCM and washed with water. The organic layer was dried over Na2S04, filtered and concentrated in vacuo to give the crude title compound (9.7 g, 98% yield) which was used without further purification. Rf = 0.49 (DCM/MeOH : 90/10).
Reference compound G: Methyl [(4S)-6-(4-chlorophenyl)-1 -methyl-8-(methyloxy)-4H- [1 ,2,4]triazolo[4,3-a][1 ,4]benz
The crude Methyl [(3S)-2-[(1 Z)-2-acetylhydrazino]-5-(4-chlorophenyl)-7-(methyloxy)-3H- 1 ,4-benzodiazepin-3-yl]acetate (for a preparation see Reference compound F) (assumed 9.7 g) was suspended in THF (100 ml) and AcOH (60 mL) was added at room temperature. The reaction mixture was stirred at this temperature for 2 days before being concentrated under reduced pressure. The crude solid was triturated in /'-Pr20 and filtered to give the title compound (8.7 g, 91 % over 3 steps) as an off-white solid.
HRMS (M+H)+ calculated for C21 H20CIN4O3 41 1.1229; found 41 1.1245.
Reference compound H: [(4S)-6-(4-Chlorophenyl)-1 -methyl-8-(methyloxy)-4H- [1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]acetic acid
To a solution of Methyl [(4S)-6-(4-chlorophenyl)-1 -methyl-8-(methyloxy)-4H- [1 ,2,4]triazolo[4,3-a][1 ,4]benzodiazepin-4-yl]acetate (for a preparation see Reference compound G)(7.4 g, 18.1 mmol) in THF (130 mL) at room temperature was added 1 N NaOH (36.2 mL, 36.2 mmol). The reaction mixture was stirred at this temperature for 5h before being quenched with 1 N HCI (36.2 mL) and concentrated in vacuo. Water is then added and the aqueous layer was extracted with DCM (x3) and the combined organic layers were dried over Na2S04, filtered and concentrated under reduced pressure to give the title compound (7 g, 98% yield) as a pale yellow solid.
PATENT
WO2014028547
http://www.nature.com/nature/journal/v468/n7327/fig_tab/nature09589_F1.html

http://www.google.com/patents/WO2014028547A1?cl=en
Zhao, Y., et al.: J. Med. Chem., 56, 7498 (2013); Mirguet, O., et al.: J. Med. Chem., 56, 7501 (2013);
| Patent ID | Date | Patent Title |
|---|---|---|
| US2015119435 | 2015-04-30 | COMPOUNDS USEFUL FOR PROMOTING PROTEIN DEGRADATION AND METHODS USING SAME |
| US8975417 | 2015-03-10 | Pyrazolopyrrolidine derivatives and their use in the treatment of disease |
| US2014356322 | 2014-12-04 | Compounds & Methods for the Enhanced Degradation of Targeted Proteins & Other Polypeptides by an E3 Ubiquitin Ligase |
| US2014243322 | 2014-08-28 | BIVALENT BROMODOMAIN LIGANDS, AND METHODS OF USING SAME |
| US2014243321 | 2014-08-28 | BIOORTHOGONAL MONOMERS CAPABLE OF DIMERIZING AND TARGETING BROMODOMAINS, AND METHODS OF USING SAME |
| US2014243286 | 2014-08-28 | BROMODOMAIN LIGANDS CAPABLE OF DIMERIZING IN AN AQUEOUS SOLUTION, AND METHODS OF USING SAME |
| US2013079335 | 2013-03-28 | Benzotriazolodiazepine Compounds Inhibitors Of Bromodomains |
| US2012252781 | 2012-10-04 | Benzodiazepine Bromodomain Inhibitor |
| US2012220573 | 2012-08-30 | Benzodiazepine Bromodomain Inhibitor |
| US2012208800 | 2012-08-16 | Bromodomain Inhibitors For Treating Autoimmune And Inflammatory Diseases |
| Patent ID | Date | Patent Title |
|---|---|---|
| US2015366877 | 2015-12-24 | METHODS OF TREATMENT OF HUMAN CYTOMEGALOVIRUS INFECTION AND DISEASES WITH BROMODOMAIN INHIBITORS |
| US2015299210 | 2015-10-22 | Benzodiazepine Bromodomain Inhibitor |
| US2015291562 | 2015-10-15 | IMIDE-BASED MODULATORS OF PROTEOLYSIS AND ASSOCIATED METHODS OF USE |
| US2015246923 | 2015-09-03 | 9H-PYRIMIDO[4,5-B]INDOLES AND RELATED ANALOGS AS BET BROMODOMAIN INHIBITORS |
| US2015238507 | 2015-08-27 | BENZODIAZEPINES FOR TREATING SMALL CELL LUNG CANCER |
| US2015210706 | 2015-07-30 | Benzodiazepine Bromodomain Inhibitor |
| US2015174138 | 2015-06-25 | DIAGNOSTIC AND TREATMENT METHODS IN SUBJECTS HAVING OR AT RISK OF DEVELOPING RESISTANCE TO CANCER THERAPY |
| US2015133436 | 2015-05-14 | Bromodomain Inhibitors For Treating Autoimmune And Inflammatory Diseases |
| US2015133434 | 2015-05-14 | Compositions and Methods for Reactivating Latent Immunodeficiency Virus |
| US2015119435 | 2015-04-30 | COMPOUNDS USEFUL FOR PROMOTING PROTEIN DEGRADATION AND METHODS USING SAME |
CCNC(=O)CC1C2=NN=C(N2C3=C(C=C(C=C3)OC)C(=N1)C4=CC=C(C=C4)Cl)C



























