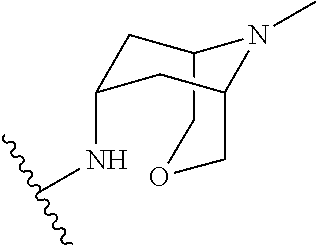
TAK-058 , ENV-8058
5-HT 3 receptor antagonist
Envoy Therapeutics, Inc.
1-(1-methyl-1H-pyrazol-4-yl)-N-((1R,5S,7S)-9-methyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-yl)-1H-indole-3-carboxamide
l-(l-methyl-lH-pyrazol-4-yl)-N-((lR,5 .7S)-9-methyl-3-oxa-9-azabicyclo[3.3.11nonan-7-yl)-lH-indole-3-carboxamide
1-(1-methyl-1H- pyrazol-4-yl)-N- ((1R,5S,7S)- 9-methyl-3- oxa-9-azabicyclo [3.3.1]nonan-7- yl)-1H-indole-3- carboxamide, 2,2,2- trifluoroacetic acid salt
N-(9-methyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-yl)-1-(1-methylpyrazol-4-yl)indole-3-carboxamide
| Molecular Formula: | C21H25N5O2 |
|---|---|
| Molecular Weight: | 379.4555 g/mol |
Phase I Schizophrenia
| Company | Takeda Pharmaceutical Co. Ltd. |
| Description | Serotonin (5-HT3) receptor antagonist |
| Molecular Target | Serotonin (5-HT3) receptor |
| Mechanism of Action | Serotonin (5-HT3) receptor antagonist |
| Therapeutic Modality | Small molecule |
| Latest Stage of Development | Phase I |
| Standard Indication | Schizophrenia |
| Indication Details | Treat schizophrenia |
- 01 Dec 2015 Phase-I clinical trials in Schizophrenia (Combination therapy) in USA (PO)
- 01 Dec 2015 Takeda completes a phase I trial in Healthy volunteers in USA (NCT02389881)
- 28 Nov 2015 Takeda plans a phase I trial in Schizophrenia (Combination therapy) in USA (NCT02614586)
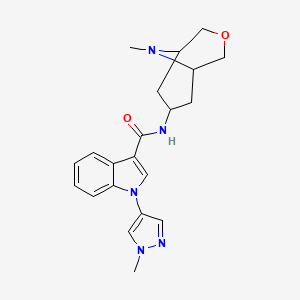

1 -( 1 -methyl- 1 H-pyrazol-4-yl)-N-((lR,5S,7S)-9-methyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-yl)-lH-indole-3-carboxamide, free base, which is an antagonist of the 5-HT3 receptor. 1 -(1 -Methyl- 1 H-pyrazol-4-yl)-N-((lR,5S,7S)-9-methyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-yl)-lH-indole-3-carboxamide, 2,2,2-trifluoroacetic acid salt, is disclosed in PCT Publication No. WO
2014/014951, published January 23, 2014.
1-(1-methyl-1H-pyrazol-4-yl)-N-((1R,5S,7S)-9-methyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-yl)-1H-indole-3-carboxamide a 5-HT3 receptor antagonist, useful for treating anxiety, depression, eating disorder, schizophrenia, cognitive dysfunction, Parkinson's disease, Huntington's Chorea, presenile dementia, Alzheimer's disease and atherosclerosis.
This compound was originally claimed in WO2014014951, Takeda, following its acquisition of Envoy Therapeutics, is developing TAK-058 (ENV-8058), a 5-HT3 receptor antagonist, as an oral solution for treating schizophrenia, especially cognitive impairment associated with schizophrenia.
In July 2015, the drug was listed as being in phase I development. TAK-058 may have emerged from a schizophrenia therapy program which used Envoy's bacTRAP translational profiling technology to identify a protein target in the brain.
PATENT
WO2014014951
Example 5
Synthesis of l-(l-methyl-lH-pyrazol-4-yl)-N-((lR,5 .7S)-9-methyl-3-oxa-9-azabicyclo[3.3.11nonan-7-yl)-lH-indole-3-carboxamide. 2.2.2-trifluoroacetic acid salt

Step 1 : methyl 1-(1 -methyl- lH-pyrazol-4-yl)-lH-indole-3-carboxylate. TFA
To a sealed tube was added copper(I) iodide (65.2 mg, 0.342 mmol), methyl 1H-indole-3-carboxylate (200 mg, 1.142 mmol) and potassium phosphate (509 mg, 2.397 mmol), then the reaction vessel was evacuated and purged with nitrogen (3x). Next, 4-bromo-l-methyl-lH-pyrazole (184 mg, 1.142 mmol) and (lR,2R)- ,N2-dimethylcyclohexane-l,2-diamine (109 μΐ, 0.685 mmol) were added, followed by toluene (1 142 μΐ). The reaction tube was evacuated and purged with nitrogen, then sealed and heated at 1 10 °C for 24 h. HPLC purification provided the title compound as a colorless oil.
Step 2: 1-(1 -methyl- lH-pyrazol-4-yl)-lH-indole-3-carboxylic acid hydrochloride
To a solution of methyl 1-(1 -methyl- lH-pyrazol-4-yl)-lH-indole-3-carboxylate, TFA
(3.5 mg, 9.48 μιηοΐ) in MeOH (95 μΐ) was added a solution of aq. KOH (33.2 μΐ, 0.066 mmol, 2 M). The reaction mixture was stirred at RT overnight, then acidified with IN HC1.
The solvent was evaporated under reduced pressure and the residue was dried under vacuum overnight. The title compound was used without further purification.
Step 3 : l-(l-methyl-lH-pyrazol-4-yl)-N-((lR,5 .7S)-9-methyl-3-oxa-9-azabicyclor3.3.11nonan-7-yl)-lH-indole-3-carboxamide, 2,2,2-trifluoroacetic acid salt
To a mixture of 1-(1 -methyl- lH-pyrazol-4-yl)-lH-indole-3-carboxylic acid hydrochloride (2.6 mg, 9.36 μιηοΐ) in DMF (187 μΐ) was added HATU (4.27 mg, 0.01 1 mmol) and DIPEA (8.18 μΐ, 0.047 mmol). After the reaction mixture was stirred at RT for 15 min, (lR,5S,7S)-9-methyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-amine, TFA (3.04 mg, 0.01 1 mmol) was added and stirring was continued for 2 h. HPLC purification afforded the title compound as a white solid. MS (ESI, pos. ion) m/z: 380.30 (M+l).
PATENT
WO-2016053947
EXAMPLE 1 : l-(l-methyl-lH-pyrazol-4-yl)-N-((lR,5S,7S)-9-methyl-3-oxa-9-azabicyclo[3.3.1 ]nonan-7-yl)- lH-indole-3-carboxamide
l-(l-Methyl-lH-pyrazol-4-yl)-lH-indole-3-carboxylic acid (128.7 g, 0.53 mol,) and anhydrous THF (645 mL) was heated to about 43°C. Oxalyl chloride (137.7 g, 92 mL, 1.08 mol) was added dropwise between 40 and 50°C. Gas evolution ceased in approximately 30 minutes. The resulting suspension was stirred for 2 hours at 50°C, allowed to cool to room temperature, and then stirred overnight. The suspension was diluted with heptane (1.5 L), stirred for 10 minutes, and allowed to settle. The supernatant was removed. The addition of heptane (1.5 L), followed by stirring, settling, and decanting was repeated two more times.
The resulting suspension was diluted with anhydrous THF (645 mL) and the ratio between THF and heptane was determined by NMR to be 3:2. The reaction mixture was cooled to 5°C and to the mixture was added DIPEA base (138 g, 1.07 mol) at such a rate that the temperature did not exceed 20°C. Next (li?,55*,7S)-9-methyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-amine (101.4 g, 0.63 mol) in 500 mL of anhydrous THF was added. The reaction mixture was warmed to ambient temperature and stirred at 20 to 23°C overnight to give a suspension.
The suspension was filtered and the cake was dissolved in IN HC1 (2.6 L). The aqueous layer was washed with EtOAc (3 x 2.6 L). The aqueous layer was cooled to 5°C and was basified to pH 12 with aqueous potassium hydroxide (230 g) solution in water (500 mL). The mixture was stirred at 5 to 10°C overnight to give a solid. The product was filtered, washed with water (2 x 1.2 L), followed by MTBE (2 x 1.2 L), and then dried to give 128 g (64%) of the (crude) title compound.
Patent
https://www.google.co.in/patents/US20140024644
1-(1-methyl-1H- pyrazol-4-yl)-N- ((1R,5S,7S)- 9-methyl-3- oxa-9-azabicyclo [3.3.1]nonan-7- yl)-1H-indole-3- carboxamide, 2,2,2- trifluoroacetic acid salt
Synthetic Procedures Reference 1 Synthesis of (1R,5S,7S)-tert-butyl 7-hydroxy-3-oxa-9-azabicyclo[3.3.1]nonane-9-carboxylate
- Sodium borohydride (259 mg, 6.84 mmol) was added portion-wise to a solution of (1R,5S)-tert-butyl 7-oxo-3-oxa-9-azabicyclo[3.3.1]nonane-9-carboxylate (550 mg, 2.279 mmol) in MeOH (4559 μl) at 0° C. After 5 min, the reaction mixture was allowed to warm to RT then stirred for 30 min. The mixture was concentrated under reduced pressure, dissolved in EtOAc and washed with brine. The combined organic layers were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to afford the title compound as a white solid, which was used without further purification.
Example 4 Synthesis of N-((1R,5S,7S)-9-methyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-yl)-1-(1H-pyrazol-4-yl)-1H-indole-3-carboxamide, 2,2,2-trifluoroacetic acid salt
- A mixture of 1-((1-benzyl-1H-pyrazol-4-yl)-N-((1R,5S,7S)-9-methyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-yl)-1H-indole-3-carboxamide 2,2,2-trifluoroacetate (85 mg, 0.149 mmol) and 10% Pd—C (120 mg) in MeOH (1.0 ml) was stirred at RT under H2 for 2 days. Filtration and concentration afforded the title compound as a white solid. MS (ESI, pos. ion) m/z: 366.20 (M+1).
- To a sealed tube was added copper(I) iodide (65.2 mg, 0.342 mmol), methyl 1H-indole-3-carboxylate (200 mg, 1.142 mmol) and potassium phosphate (509 mg, 2.397 mmol), then the reaction vessel was evacuated and purged with nitrogen (3×). Next, 4-bromo-1-methyl-1H-pyrazole (184 mg, 1.142 mmol) and (1R,2R)—N1,N2-dimethylcyclohexane-1,2-diamine (109 μl, 0.685 mmol) were added, followed by toluene (1142 μl). The reaction tube was evacuated and purged with nitrogen, then sealed and heated at 110° C. for 24 h. HPLC purification provided the title compound as a colorless oil.
- To a solution of methyl 1-(1-methyl-1H-pyrazol-4-yl)-1H-indole-3-carboxylate, TFA (3.5 mg, 9.48 μmol) in MeOH (95 μl) was added a solution of aq. KOH (33.2 μl, 0.066 mmol, 2 M). The reaction mixture was stirred at RT overnight, then acidified with 1N HCl. The solvent was evaporated under reduced pressure and the residue was dried under vacuum overnight. The title compound was used without further purification.
- To a mixture of 1-(1-methyl-1H-pyrazol-4-yl)-1H-indole-3-carboxylic acid hydrochloride (2.6 mg, 9.36 μmol) in DMF (187 μl) was added HATU (4.27 mg, 0.011 mmol) and DIPEA (8.18 μl, 0.047 mmol). After the reaction mixture was stirred at RT for 15 min, (1R,5S,7S)-9-methyl-3-oxa-9-azabicyclo[3.3.1]nonan-7-amine, TFA (3.04 mg, 0.011 mmol) was added and stirring was continued for 2 h. HPLC purification afforded the title compound as a white solid. MS (ESI, pos. ion) m/z: 380.30 (M+1).
| 15 | TFA |
| 379.456 MW | 380.30 MS +1 |
C12CC(CC(N1C)COC2)NC(c4c3ccccc3n(c4)c5cnn(c5)C)=O
CN1C=C(C=N1)N2C=C(C3=CC=CC=C32)C(=O)NC4CC5COCC(C4)N5C