Altiratinib
DCC-2701; DP-5164
CAS :1345847-93-9
N-[4-({2-[(cyclopropylcarbonyl)amino]pyridin-4-yl}oxy)-2,5-difluorophenyl]-N-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide
N-(4-((2-(cyclopropanecarboxamido)pyridin-4-yl)oxy)-2,5-difluorophenyl)-N-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide
Mechanism of Action:MET/TIE2/VEGFR2/TRK (A,B,C) kinase inhibitorIndication:invasive solid tumors. The FDA has granted altiratinib Orphan Drug Designation for glioblastoma multiforme (GBM)
Development Stage:Phase I
Developer:Deciphera Pharmaceuticals, Llc
%20structure.gif)
Altiratinib, also known as DCC-270, DP-5164, is an oral, selective and highly potent inhibitor of MET, TIE2, VEGFR2 and TRK kinases with potential anticancer activity. DCC-2701 effectively reduces tumor burden in vivo and blocks c-MET pTyr(1349)-mediated signaling, cell growth and migration as compared with a HGF antagonist in vitro. Importantly, DCC-2701's anti-proliferative activity was dependent on c-MET activation induced by stromal human fibroblasts and to a lesser extent exogenous HGF. DCC-2701 may be superior to HGF antagonists that are in clinical trials and that pTyr(1349) levels might be a good indicator of c-MET activation and likely response to targeted therapy as a result of signals from the microenvironment. Inhibition of MET kinase blocks a key mechanism in tumor cells that causes cancer invasiveness and metastasis. (Oncogene. 2015 Jan 8;34(2):144-53.)
DCC-2701 is an angiogenesis inhibitor acting on Tie2 receptor, HGF receptor and VEGFR-2. The product is being evaluated in phase I clinical studies at Deciphera for the oral treatment of solid tumors.
Altiratinib(DCC-2701) is a novel c-MET/TIE-2/VEGFR inhibitor; effectively reduce tumor burden in vivo and block c-MET pTyr(1349)-mediated signaling, cell growth and migration as compared with a HGF antagonist in vitro.


SYNTHESIS
CLICK ON IMAGE TO ENLARGEPATENT
https://www.google.co.in/patents/WO2011137342A1?cl=en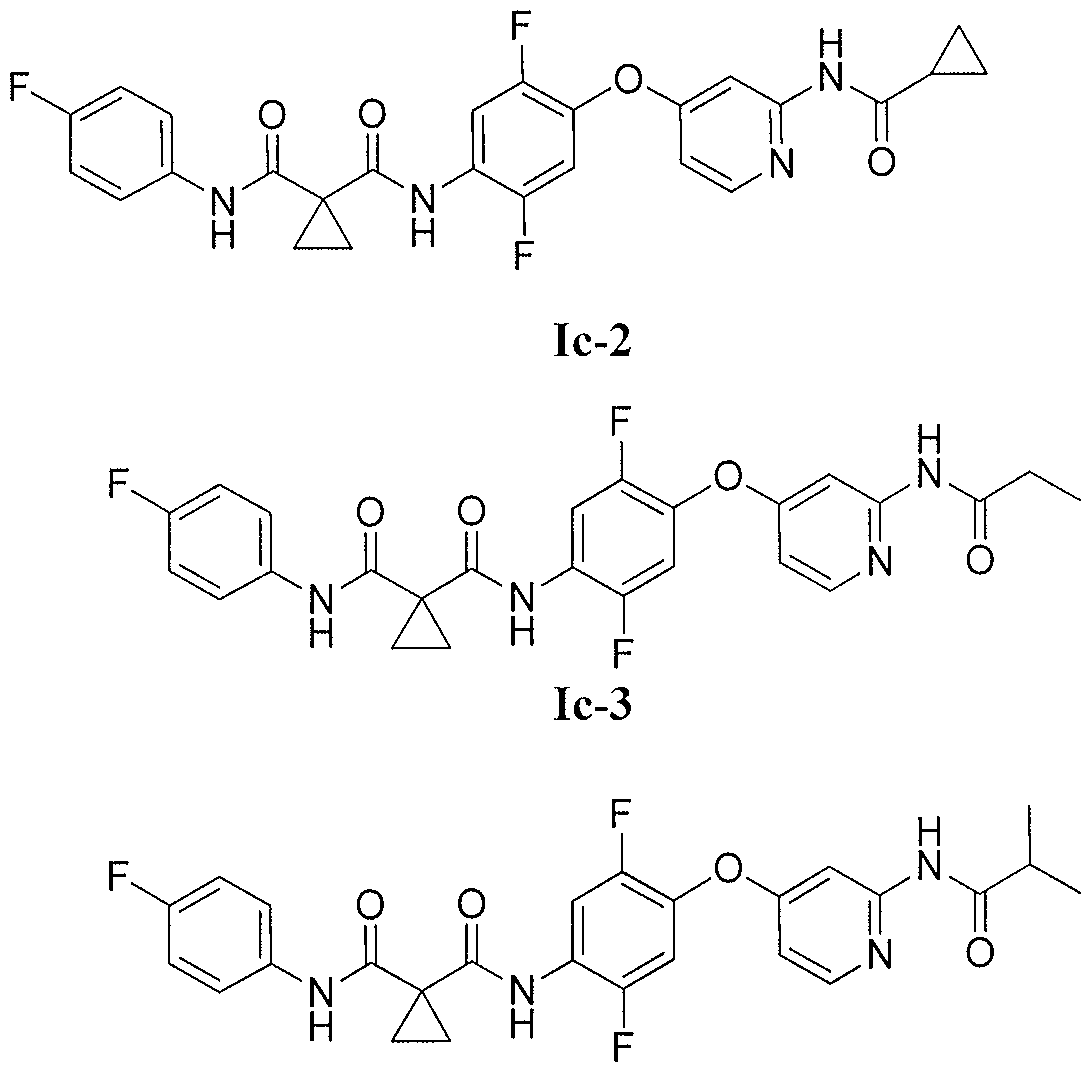
Scheme 1
BEAWARE THIS IS NOT THE COMPD
Scheme 11INTERMEDIATES
Scheme 9[00108] A non-limiting example of Scheme 9 is illustrated below for the synthesis of 36, a specific example of 26 wherein X is F, Y is CI, and Zl , Z2, and Z3 are CH (Scheme 10). Addition of l ,2,4-trifluoro-5-nitrobenzene (33) to a solution of 2-chloropyridin-4-ol (34) and sodium hydride in DMF at 0 °C yields the nitro intermediate 35. The nitro moiety of 35 is subsequently reduced at RT in the presence of zinc dust and ammonium chloride in solution of me hanol and THF to yield amine 36.
Scheme 10
[00109] A non-limiting example of Scheme 7 is illustrated in Scheme 11, beginning with intermediate 36, prepared in Scheme 10. Thus, 36 readily reacts with acid chloride 13 (see Scheme 3) in the presence of triethylamine to yield chloro-pyridine 37. Chloro-pyridine 37 is then converted to 38, a specific example of 1 wherein Rl is F, X is F, Zl , Z2, and Z3 are CH and R3 is -C(0)CH3, upon treatment with acetamide (an example of R3-NH2 27 where R3 is acetyl) and cesium carbonate in the presence of a catalytic amount of palladium acetate and xantphos.
NOTE 38 IS NOT THE FINAL PRODUCT
REFERENCES
Kwon Y, Smith BD, Zhou Y, Kaufman MD, Godwin AK. Effective inhibition of c-MET-mediated signaling, growth and migration of ovarian cancer cells is influenced by the ovarian tissue microenvironment. Oncogene. 2015 Jan 8;34(2):144-53. doi: 10.1038/onc.2013.539. Epub 2013 Dec 23. PubMed PMID: 24362531; PubMed Central PMCID: PMC4067476.SEEhttp://newdrugapprovals.org/2015/10/09/altiratinib/
////Altiratinib, DCC-2701, DP-5164, Phase I, Deciphera Pharmaceuticals






