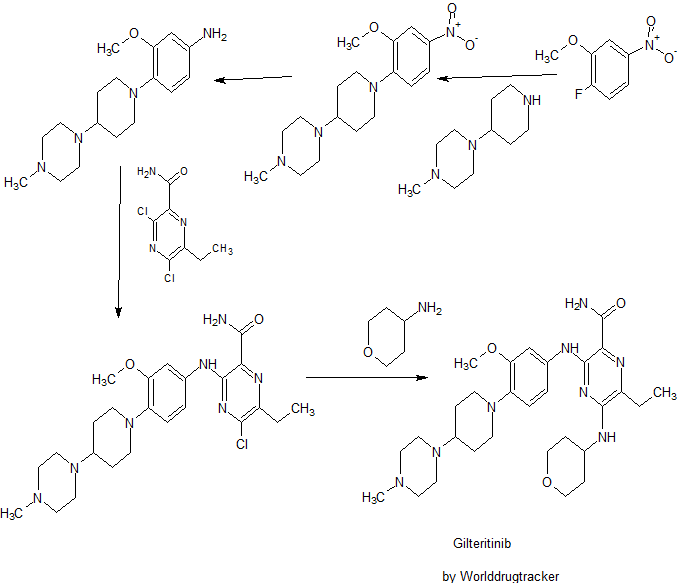
Gilteritinib
ASP-2215
Treatment of Acute Myeloid Leukemia
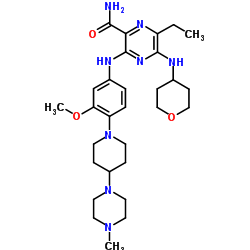
6-ethyl-3-{3-methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]anilino}-5-[(oxan-4-yl)amino]pyrazine-2-carboxamide
C29H44N8O3, 552.71
Phase III
A FLT3/AXL inhibitor potentially for the treatment of acute myeloid leukemia.
CAS No. 1254053-43-4
| Astellas Pharma INNOVATOR | |
| Mechanism Of Action | Axl receptor tyrosine kinase inhibitors, Fms-like tyrosine kinase 3 inhibitors, Proto oncogene protein c-kit inhibitors |
|---|---|
| Who Atc Codes | L01X-E (Protein kinase inhibitors) |
| Ephmra Codes | L1H (Protein Kinase Inhibitor Antineoplastics) |
| Indication | Cancer, Hepatic impairment |
IC50 value: 0.29 nM(FLT3); <1 nM(Axl kinase)
Target: FLT3/AXL inhibitor
ASP2215 inhibited the growth of MV4-11 cells, which harbor FLT3-ITD, with an IC50 value of 0.92 nM, accompanied with inhibition of pFLT3, pAKT, pSTAT5, pERK, and pS6. ASP2215 decreased tumor burden in bone marrow and prolonged the survival of mice intravenously transplanted with MV4-11 cells. ASP2215 may have potential use in treating AML.
SYNTHESIS
PATENT
WO 2010128659
Patent
Compound A is 6-ethyl-3 - ({3-methoxy-4- [4- (4-methylpiperazin-1-yl) piperidin-1-yl] phenyl} amino) -5- a (tetrahydro -2H- pyran-4-ylamino) pyrazine-2-carboxamide, its chemical structure is shown below.
[Formula 1]

[Formula 1]
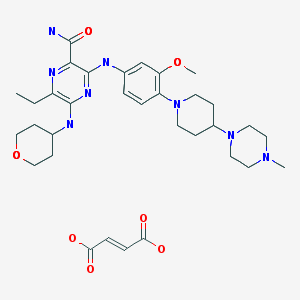
Gilteritinib fumarate
Gilteritinib fumarate [USAN]
RN: 1254053-84-3
UNII: 5RZZ0Z1GJT
2-Pyrazinecarboxamide, 6-ethyl-3-((3-methoxy-4-(4-(4-methyl-1-piperazinyl)-1-piperidinyl)phenyl)amino)-5-((tetrahydro-2H-pyran-4-yl)amino)-, (2E)-2-butenedioate (2:1)
- ASP-2215 hemifumarate
- Molecular Formula, 2C29-H44-N8-O3.C4-H4-O4, Molecular Weight, 1221.5108
Astellas Inititaties Phase 3 Registration Trial of gilteritinib (ASP2215) in Relapsed or Refractory Acute Myeloid Leukemia Patients
TOKYO, Japan I October 28, 2015 I Astellas Pharma Inc. (TSE:4503) today announced dosing of the first patient in a randomized Phase 3 registration trial of gilteritinib (ASP2215)versus salvage chemotherapy in patients with relapsed or refractory (R/R) acute myeloid leukemia (AML). The primary endpoint of the trial is overall survival (OS).
Gilteritinibis a receptor tyrosine kinase inhibitor of FLT3 and AXL, which are involved in the growth of cancer cells. Gilteritinibhas demonstrated inhibitory activity against FLT3 internal tandem duplication (ITD) as well as tyrosine kinase domain (TKD), two common types of FLT3 mutations that are seen in up to one third of patients with AML.
The gilteritinib Phase 3 trial follows a Phase 1/2 trial, which evaluated doses from 20 to 450 mg once daily. A parallel multi-dose expansion cohort was initiated based on the efficacy seen in the dose escalation phase. Preliminary data from the Phase 1/2 trial presented at the 2015 American Society of Clinical Oncology annual meeting demonstrated a 57.5 percent overall response rate and a 47.2 percent composite Complete Response (CR) rate (CR + CR with incomplete platelet recovery + CR with incomplete hematologic recovery) in 106 patients with FLT3 mutations who received 80 mg and higher doses. Median duration of response was 18 weeks across all doses and median OS was approximately 27 weeks at 80 mg and above in FLT3 mutation positive patients. Common drug-related adverse events (> 10%) observed in the study were diarrhea (13.4%), fatigue (12.4%) and AST increase (11.3%). At the 450 mg dose, two patients reached dose-limiting toxicity (grade 3 diarrhea and ALT/AST elevation) and the maximum tolerated dose was determined to be 300 mg.
On October 27, 2015, the Japanese Ministry of Health, Labor and Welfare (MHLW) announced the selection of gilteritinib as one of the first products designated for SAKIGAKE.
About the Phase 3 Study
The Phase 3 trial is an open-label, multicenter, randomized study of gilteritinib versus salvage chemotherapy in patients with Acute Myeloid Leukemia (AML). The study will enroll 369 patients with FLT3 activating mutation in bone marrow or whole blood, as determined by central lab, AML who are refractory to or have relapsed after first-line AML therapy. Subjects will be randomized in a 2:1 ratio to receive gilteritinib (120 mg) or salvage chemotherapy consisting of LoDAC (low-dose cytarabine), azacitidine, MEC (mitoxantrone, etoposide, and intermediate-dose cytarabine), or FLAG-IDA (fludarabine, cytarabine, and granulocyte colony-stimulating factor with idarubicin). The primary endpoint of the trial is OS. For more information about this trial go to www.clinicaltrials.gov, trial identifier NCT02421939.
Gilteritinib was discovered through a research collaboration with Kotobuki Pharmaceutical Co., Ltd., and Astellas has exclusive global rights to develop, manufacture and potentially commercialize gilteritinib.
About Acute Myeloid Leukemia
Acute myeloid leukemia is a cancer that impacts the blood and bone marrow and most commonly experienced in older adults. According to the//www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf” target=”_blank” rel=”nofollow”>American Cancer Society, in 2015, there will be an estimated 20,830 new cases of AML diagnosed in the United States, and about 10,460 cases will result in death.
About SAKIGAKE
The SAKIGAKE designation system can shorten the review period in the following three approaches: 1.) Prioritized Consultation 2.) Substantial Pre-application Consultation and 3.) Prioritized Review. Also, the system will promote development with the following two approaches: 4.) Review Partner System (to be conducted by the Pharmaceuticals and Medical Devices Agency) and 5.) Substantial Post-Marketing Safety Measures.
About Astellas
Astellas Pharma Inc., based in Tokyo, Japan, is a company dedicated to improving the health of people around the world through the provision of innovative and reliable pharmaceutical products. We focus on Urology, Oncology, Immunology, Nephrology and Neuroscience as prioritized therapeutic areas while advancing new therapeutic areas and discovery research leveraging new technologies/modalities. We are also creating new value by combining internal capabilities and external expertise in the medical/healthcare business. Astellas is on the forefront of healthcare change to turn innovative science into value for patients. For more information, please visit our website at www.astellas.com/en.
SOURCE: Astellas Pharma
////////1254053-43-4, Gilteritinib, ASP-2215, PHASE 3, ASP 2215, Astellas Pharma, Acute Myeloid Leukemia
CCc1c(nc(c(n1)C(=O)N)Nc2ccc(c(c2)OC)N3CCC(CC3)N4CCN(CC4)C)NC5CCOCC5
CCc1c(nc(c(n1)