ND 0126
CAS 1240322-54-6
| Molecular Formula: | C29H25F3N6O3 |
|---|---|
| Molecular Weight: | 562.54241 g/mol |
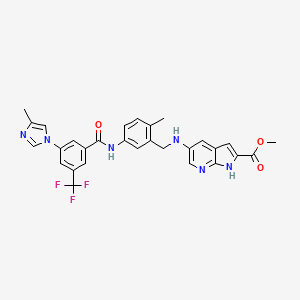
methyl 5-[[2-methyl-5-[[3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)benzoyl]amino]phenyl]methylamino]-1H-pyrrolo[2,3-b]pyridine-2-carboxylate
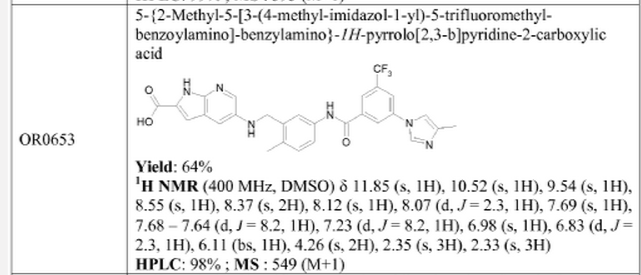
5-{2-Methyl-5-[3-(4-methyl-imidazol-1-yl)-5-trifluoromethyl-benzoylamino]-benzylamino}-1H-pyrrolo[2,3-b]pyridine-2-carboxylic Acid Methyl Ester
Potent dual ABL/SRC inhibitors based on a 7-azaindole core with the aim of developing compds. that demonstrate a wider activity on selected oncogenic kinases. Multi-Targeted Kinase Inhibitors (MTKIs) were then derived, focusing on kinases involved in both angiogenesis and tumorigenesis processes.
Dysfunction/deregulation of protein kinases (PK) is the cause of a large number of pathologies including oncological, immunological, neurological, metabolic and infectious diseases. This has generated considerable interest in the development of small molecules and biological kinase inhibitors for the treatment of these disorders.
Numerous PK are particularly deregulated during the process of tumorigenesis. Consequently protein kinases are attractive targets for anticancer drugs, including small molecule inhibitors that usually act to block the binding of ATP or substrate to the catalytic domain of the tyrosine kinase and monoclonal antibodies that specifically target receptor tyrosine kinases (RTK) and their ligands. In solid malignancies, it is unusual for a single kinase abnormality to be the sole cause of disease and it is unlikely that tumors are dependent on only one abnormally activated signaling pathway. Instead multiple signaling pathways are dysregulated. Furthermore, even single molecular abnormalities may have multiple downstream effects. Multi targeted therapy using a single molecule (MTKI = "Multi-Targeted Kinase Inhibitors") which targets several signaling pathways simultaneously, is more effective than single targeted therapy. Single targeted therapies have shown activity for only a few indications and most solid tumors show deregulation of multiple signaling pathways. For example, the combination of a vascular endothelial growth factor receptor (VEGFR) inhibitor and platelet derived growth factor receptor (PDGFR) inhibitor results in a cumulative antitumor efficacy (Potapova et al, Mol Cancer Ther 5, 1280-1289, 2006).
Tumors are not built up solely of tumor cells. An important part consists of connective tissue or stroma, made up of stromal cells and extracellular matrix, which is produced by these cells. Examples of stromal cells are fibroblasts, endothelial cells and macrophages. Stromal cells also play an important role in the carcinogenesis, where they are characterized by upregulation or induction of growth factors and their receptors, adhesion molecules, cytokines, chemokines and proteolytic enzymes (Hofmeister et al., Immunotherapy 57, 1-17, 2007; Raman et al, Cancer Letters 256, 137-165, 2007; Fox et al, The Lancet Oncology 2, 278-289, 2001) The receptor associated tyrosine kinase VEGFR on endothelial and tumor cells play a central role in the promotion of cancer by their involvement in angiogenesis (Cebe-Suarez et al, Cell Mol Life Sci 63, 601-615, 2006). In addition, the growth factors TGF-β, PDGF and FGF2 secreted by cancer cells transform normal fibroblasts into tumor associated fibroblasts, which make their receptors a suitable target for inhibition by kinase inhibitors (Raman et al, 2007).
Moreover, increasing evidence suggests a link between the EGF receptor (EGFR) and HER2 pathways and VEGF-dependent angiogenesis and preclinical studies have shown both direct and indirect angiogenic effects of EGFR signaling (Pennell and Lynch, The Oncologist 14, 399-411, 2009). Upregulation of tumor pro -angiogenic factors and EGFR- independent tumor-induced angiogenesis have been suggested as a potential mechanism by which tumor cells might overcome EGFR inhibition. The major signaling pathways regulated by EGFR activation are the PI3K, MAPK and Stat pathways that lead to increased cell proliferation, angiogenesis, inhibition of apoptosis and cell cycle progression. EGFR is overexpressed in a wide variety of solid tumors, such as lung, breast, colorectal and cancers of the head and neck (Cook and Figg, CA Cancer J Clin 60, 222-243 2010). Furthermore, higher expression of EGFR has been shown to be associated with metastasis, decreased survival and poor prognosis.
c-Src, a membrane-associated non receptor tyrosine kinase, is involved in a number of important signal transduction pathways and has pleiotropic effects on cellular function. c-Src integrates and regulates signaling from multiple transmembrane receptor-associated tyrosine kinases, such as the EGFR, PDGFR, IGF1R, VEGFR, HER2. Together, these actions modulate cell survival, proliferation, differentiation, angiogenesis, cell motility, adhesion, and invasion (Brunton and Frame, Curr Opin Pharmacol 8, 427-432, 2008). Overexpression of the protein c-Src as well as the increase in its activity were observed in several types of cancers including colorectal, gastrointestinal (hepatic, pancreatic, gastric and oesophageal), breast, ovarian and lung (Yeatman, Nat Rev Cancer 4, 470-480, 2004).
The activation in EGFR or KRAS in cancers leads to a greatly enhanced level of Ras- dependent Raf activation. Hence, elimination of Raf function is predicted to be an effective treatment for the numerous cancers initiated with EGFR and KRAS lesions (Khazak et al, Expert Opin. Ther. Targets 11, 1587-1609, 2007). Besides activation of Raf signaling in tumors, a number of studies implicate the activation of the Ras-Raf-MAPK signaling pathway as a critical step in vasculo genesis and angiogenesis. Such activation is induced by growth factor receptors such as VEGFR2, FGFR2 and thus inhibition of Raf activation represents a legitimate target for modulation of tumor angiogenesis and vascularization.
Although VEGFR, PDGFR, EGFR, c-Src and Raf are important targets on both tumor cells and tumor stroma cells, other kinases such as FGFR only function in stromal cells and other oncogenes often only function in tumor cells.
Protein kinases are fundamental components of diverse signaling pathways, including immune cells. Their essential functions have made them effective therapeutic targets. Initially, the expectation was that a high degree of selectivity would be critical; however, with time, the use of "multikinase" inhibitors has expanded. Moreover, the spectrum of diseases in which kinase inhibitors are used has also expanded to include not only malignancies but also immune-mediated diseases / inflammatory diseases. The first step in signaling by multi-chain immune recognition receptors is mediated initially by Src family protein tyrosine kinases. MTKI targeting kinases involved in immune function are potential drugs for autoimmune diseases such as rheumatoid arthritis, psoriasis and inflammatory bowel diseases (Kontzias et al. , F 1000 Medicine Reports 4, 2012)
Protein kinases mentioned previously are also key components of many other physiological and pathological mechanisms such as neurodegeneration and neuroprotection (Chico et al, Nature Reviews Drug Discovery 8, 892-909, 2009), atherosclerosis, osteoporosis and bone resorption, macular degeneration, pathologic fibrosis, Cystogenesis (human autosomal dominant polycystic kidney disease...).
In WO2010/092489 and related patents/patent applications, we identified several compounds which exhibited interesting properties for such applications. However, we have discovered that some of these compounds could be enhanced in their properties by selectively working on particular regions of their structures. However, the mechanism of action of these structures on kinases was not precisely elucidated at the time of WO2010/092489's filing and thus it was unexpectedly that we found the high activities of the structures disclosed in the present application. The subject matter of the present invention is to offer novel multi-targeted kinase inhibitors, having an original backbone, which can be used therapeutically in the treatment of pathologies associated with deregulation of protein kinases including tumorigenesis, human immune disorders, inflammatory diseases, thrombotic diseases, neurodegenerative diseases, bone diseases, macular degeneration, fibrosis, cystogenesis. The inhibitors of the present invention can be used in particular for the treatment of numerous cancers and more particularly in the case of liquid tumors such hematological cancers (leukemias) or solid tumors including but not limited to squamous cell cancer, small- cell lung cancer, non-small cell lung cancer, gastric cancer, pancreatic cancer, glial cell tumors such as glioblastoma and neurofibromatosis, cervical cancer, ovarian cancer, liver cancer, bladder cancer, breast cancer, melanoma, colorectal cancer, endometrial carcinoma, salivary gland carcinoma, renal cancer, prostate cancer, vulval cancer, thyroid cancer, sarcomas, astrocytomas, and various types of hyperproliferative diseases.
Efforts were made to improve a series of potent dual ABL/SRC inhibitors based on a 7-azaindole core with the aim of developing compounds that demonstrate a wider activity on selected oncogenic kinases. Multi-targeted kinase inhibitors (MTKIs) were then derived, focusing on kinases involved in both angiogenesis and tumorigenesis processes. Antiproliferative activity studies using different cellular models led to the discovery of a lead candidate (6z) that combined both antiangiogenic and antitumoral effects. The activity of 6z was assessed against a panel of kinases and cell lines including solid cancers and leukemia cell models to explore its potential therapeutic applications. With its potency and selectivity for oncogenic kinases, 6z was revealed to be a focused MTKI that should have a bright future in fighting a wide range of cancers.
5-{2-Methyl-5-[3-(4-methyl-imidazol-1-yl)-5-trifluoromethyl-benzoylamino]-benzylamino}-1H-pyrrolo[2,3-b]pyridine-2-carboxylic Acid Methyl Ester (6z)
The reaction was carried out as described in general procedure A using 4a (170 mg, 0.63 mmol), 3-(4-methyl-imidazol-1-yl)-5-trifluoromethyl-benzoic acid 5z (200 mg, 0.63 mmol), HATU (735 mg, 1.93 mmol), DIEA (0.56 mL, 3.22 mmol), and anhydrous DMF (16 mL). Purification by flash chromatography on silica gel (EtOAc/EtOH, 100/0 to 90/10) yielded 6z (108 mg, 30%).
1H NMR (300 MHz, DMSO-d6, δ) 12.05 (s, 1H), 10.41 (s, 1H), 8.42–8.34 (m, 2H), 8.20 (s, 1H), 8.16–8.04 (m, 2H), 7.670–7.62 (m, 3H), 7.22 (d, J = 8.2 Hz, 1H), 6.97 (d, J = 2.3 Hz, 1H), 6.90 (d, J = 1.9 Hz, 1H), 6.11 (t, J = 5.0 Hz, 1H), 4.25 (d, J = 5.0 Hz, 2H), 3.83 (s, 3H), 2.34 (s, 3H), 2.17 (s, 3H). MS (ESI) m/z 563.2 [M + H]+ and 561.2 [M – H]−.
Rational Design, Synthesis, and Biological Evaluation of 7-Azaindole Derivatives as Potent Focused Multi-Targeted Kinase Inhibitors
OriBase Pharma, Cap Gamma, Parc Euromédecine, 1682 rue de la Valsière, CS 17383, Montpellier 34189 CEDEX 4,France
J. Med. Chem., Article ASAP
DOI: 10.1021/acs.jmedchem.6b00087
Publication Date (Web): March 24, 2016
Copyright © 2016 American Chemical Society
*E-mail: ayasri@oribase-pharma.com. Phone: (+33) 467 727 670.
PATENT
WO 2010092489
Example 91: Preparation of methyl 5-(5-(3-(trifluoromethγl)-5~(4-methyl-1 H-imidazol-1 - yl)benzamido)-2-methγlbenzylamino)-1H-pyrrolo[2,3-blpyridine-2-carboχylate (ND0126)
Step 1 : preparation of methyl 5-(3-(trifluoromethyl)-5-(4-methyl-1 H-imidazol-1 - yl)benzamido)-2-methylbenzoate
The compound is obtained using the procedures of example 88 (step 4) replacing the 4-((3-(dimethylamino)pyrrolidin-1-yl)methyl)-3-(trifluoromethyl)-benzoic acid
(Shakespeare W. C, WO2007133562) by the 3-(trifluoromethyI)-5-(4-methyl-1H- imidazol-1-yl)benzoic acid.
Step 2: preparation of 3-(tπϊluoromethyl)-N-(3-formyl-4-methylphenyl)-5-(4- methyl-1H-imidazol-1-yl)benzamide
The compound is obtained by using the procedures of examples 83 (steps 1 and 2) replacing the methyl 5-(4-((4-methylpiperazin-1-yl)methyl)benzamido)-2- methylbenzoate with the methyl 5-(3-(trifluorometny))-5-(4-metbyl-1H-imidazol-1- yl)benzamido)-2-methylbenzoate.
Step 3: preparation of methyl 5-(5-(3-(trifluoromethyl)-5-(4-methyl-1 H-imidazol- 1-yl)benzamido)-2-methylbenzylamino)-1H-pyrrolo[2,3-bJpyridine-2-carboxylate (ND0126)
The composed is obtained according to example 83 (step 3) replacing N-(3-formyl-4- methylphenyl)-4-((4-methylpiperazin~1-yl)methyl)-benzamide with the 3- (trifluoromethyl)-N-(3-formyl-4-methylphenyl)-5-(4-methyl-1 H-imidazol-1-yl)benzamide.
PATENT
REFERENCES
| WO2005063747A1 * | Dec 23, 2004 | Jul 14, 2005 | Pfizer Italia S.R.L. | PYRROLO[2,3-b] PYRIDINE DERIVATIVES ACTIVE AS KINASE INHIBITORS, PROCESS FOR THEIR PREPARATION AND PHARMACEUTICAL COMPOSITION COMPRISING THEM |
| WO2008028617A1 * | Sep 4, 2007 | Mar 13, 2008 | F. Hoffmann-La Roche Ag | Heteroaryl derivatives as protein kinase inhibitors |
| WO2008124849A2 * | Apr 10, 2008 | Oct 16, 2008 | Sgx Pharmaceuticals, Inc. | Pyrrolo-pyridine kinase modulators |
| WO2008144253A1 * | May 9, 2008 | Nov 27, 2008 | Irm Llc | Protein kinase inhibitors and methods for using thereof |
| WO2014102376A1 * | Dec 30, 2013 | Jul 3, 2014 | Oribase Pharma | Protein kinase inhibitors |
| WO2014102377A1 * | Dec 30, 2013 | Jul 3, 2014 | Oribase Pharma | Azaindole derivatives as multi kinase inhibitors |
| WO2014102378A1 * | Dec 30, 2013 | Jul 3, 2014 | Oribase Pharma | Azaindole derivatives as inhibitors of protein kinases |
| US20150353540 * | Dec 30, 2013 | Dec 10, 2015 | Oribase Pharma | Azaindole derivatives as inhibitors of protein kinases |
| US2011312959 | 2011-12-22 | Derivatives of Azaindoles as Inhibitors of Protein Kinases ABL and SRC |
///////ND 0126, 1240322-54-6, PRECLINICAL
O=C(OC)c1cc2cc(cnc2n1)NCc3cc(ccc3C)NC(=O)c4cc(cc(c4)n5cc(C)nc5)C(F)(F)F
CC1=C(C=C(C=C1)NC(=O)C2=CC(=CC(=C2)N3C=C(N=C3)C)C(F)(F)F)CNC4=CN=C5C(=C4)C=C(N5)C(=O)OC