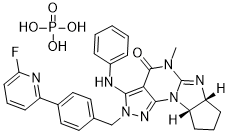
ITI 214
IC200214; ITI-214
(6aR,9aS)-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-3-(phenylamino)-5,6a,7,8,9,9a-hexahydrocyclopenta[4,5]imidazo[1,2-a]pyrazolo[4,3-e]pyrimidin-4-(2H)-one phosphate
(6aR,9aS)-5-methyl-3-(phenylamino)-2-(4-(6-fluoropyridin-2-yl)-benzyl)-5,6a,7,8,9,9a-hexahydrocyclopent[4,5]imidazo[1,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one...BASE
CAS: 1642303-38-5 (phosphate);
1160521-50-5 (free base).
Chemical Formula: C29H29FN7O5P
Molecular Weight: 605.5672
Molecular Weight: 605.5672
ITI-214 is an orally active, potent and Selective Inhibitors of Phosphodiesterase 1 for the Treatment of Cognitive Impairment Associated with Neurodegenerative and Neuropsychiatric Diseases. ITI-214 exhibited picomolar inhibitory potency for PDE1, demonstrated excellent selectivity against all other PDE families, and showed good efficacy in vivo. Currently, this investigational new drug is in Phase I clinical development and being considered for the treatment of several indications including cognitive deficits associated with schizophrenia and Alzheimer's disease, movement disorders, attention deficit and hyperactivity disorders, and other CNS and non-CNS disorders.
- Phase I Cognition disorders
- OriginatorIntra-Cellular Therapies
- ClassAntiparkinsonians; Nootropics; Small molecules
- Mechanism of ActionType 1 cyclic nucleotide phosphodiesterase inhibitors
- 21 Sep 2015Takeda completes a phase I bioavailability trial in Cognition disorders in Japan
- 21 Sep 2015Takeda completes a phase I trial in Cognition disorders in Japan
- 21 Sep 2015Takeda initiates enrolment in a phase I bioavailability trial for Cognition disorders in Japan before September 2015
Phosphodiesterase-1 (PDE-1) inhibitor
which is a picomolar PDE1 inhibitor with excellent selectivity against other PDE family members and against a panel of enzymes, receptors, transporters, and ion channels.
It is disclosed in WO 2009/075784 (U.S. Pub. No. 2010/0273754). This compound has been found to be a potent and selective phosphodiesterase 1 (PDE 1) inhibitor useful for the treatment or prophylaxis of disorders characterized by low levels of cAMP and/or cGMP in cells expressing PDE1, and/or reduced dopamine Dl receptor signaling activity (e.g., Parkinson's disease, Tourette's Syndrome, Autism, fragile X syndrome, ADHD, restless leg syndrome, depression, cognitive impairment of schizophrenia, narcolepsy); and/or any disease or condition that may be ameliorated by the enhancement of progesterone signaling. This list of disorders is exemplary and not intended to be exhaustive.

PATENT
WO 2013192556
The method of making the Compound (ea^^a^-S^a ^^^a-hexahydro-S- methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)- cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one is generally described in WO 2009/075784, the contents of which are incorporated by reference in their entirety. This compound can also be prepared as summarized or similarly summarized in the following
CMU PCU PHU PPU (SM2)
In particular, (6aR,9aS)-3-chloro-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl- 5,6a,7,8,9,9a-hexahydrocyclopenta[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)- one may be prepared as described or similarly described below.
PATENT
1 1. A compound according to claim 1 , wherein said compound is
EXAMPLE 14
(6aJ?,9aS)-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6- fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]iinidazo[l,2-fl]pyrazolo[4,3- e]pyrimidin-4(2//)-one
This compound may be made using similar method as in example 13 wherein 2-(4-(bromomethyl)phenyl)-6-fluoropyridine may be used instead of 2-(4- (dibromomethyl)phenyl)-5-fluoropyridine.
(6aJ?,9aS)-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6- fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]iinidazo[l,2-fl]pyrazolo[4,3- e]pyrimidin-4(2//)-one
This compound may be made using similar method as in example 13 wherein 2-(4-(bromomethyl)phenyl)-6-fluoropyridine may be used instead of 2-(4- (dibromomethyl)phenyl)-5-fluoropyridine.
PATENT
WO 2014205354

EXAMPLES
The method of making the Compound (ea^^a^-S^a ^^^a-hexahydro-S-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one is generally described in WO 2009/075784, the contents of which are incorporated by reference in their entirety. This compound can also be prepared as summarized or similarly summarized in the following

CMU PCU PHU PPU (SM2)

In particular, (6aR,9aS)-3-chloro-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-5,6a,7,8,9,9a-hexahydrocyclopenta[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one (Int-5) may be prepared as described or similarly described below. The free base crystals and the mono-phosphate salt crystals of the invention may be prepared by using the methods described or similarly described in Examples 1-14 below.
Preparation of (6aR,9aS)-3-chloro-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-5,6a,7,8,9,9a-hexahydrocyclopenta[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one
(4-(6-fluoropyridin-2-yl)phenyl)methanol

The mixture of Na2C03 (121 g), water (500 mL), THF (650 mL), PdCl2(PPh3)2 (997 mg), 2-bromo-6-fluoropyridine (100 g) and 4-(hydroxymethyl)phenylboronic acid (90.7 g) is stirred at 65°C for 4 h under the nitrogen atmosphere. After cooling to room temperature, THF (200 mL) is added. The organic layer is separated and washed with 5% NaCl solution twice. The organic layer is concentrated to 400 mL. After the addition of toluene (100 mL), heptane (500 mL) is added at 55°C. The mixture is cooled to room temperature. The crystals are isolated by filtration, washed with the mixture of toluene (100 mL) and heptane (100 mL) and dried to give (4-(6-fluoropyridin-2-yl)phenyl)methanol (103 g). ]H NMR (500 MHz, CDC13) δ 1.71-1.78 (m, 1H), 4.74-4.79 (m, 2H), 6.84-6.88 (m, 1H), 7.44-7.50 (m, 2H), 7.61-7.65 (m, 1H), 7.80-7.88 (m, 1H), 7.98-8.04 (m, 2H).
2-(4-(chloromethyl)phenyl)-6-fluoropyridine

The solution of thionylchloride (43.1 mL) in AcOEt (200 mL) is added to the mixture of (4-(6-fluoropyridin-2-yl)phenyl)methanol (100 g), DMF (10 mL) and AcOEt (600 mL) at room temperature. The mixture is stirred at room temperature for 1 h. After cooling to 10°C, 15% Na2C03 solution is added. The organic layer is separated and washed with water (500 mL) and 5% NaCl solution (500 mL) twice. The organic layer is concentrated to 500 mL. After the addition of EtOH (500 mL), the mixture is concentrated to 500 mL. After addition of EtOH (500 mL), the mixture is concentrated to 500 mL. After the addition of EtOH (500 mL), the mixture is concentrated to 500 mL. After addition of EtOH (200 mL), water (700 mL) is added at 40°C. The mixture is stirred at room temperature. The crystals are isolated by filtration and dried to give 2-(4-(chloromethyl)phenyl)-6-fluoropyridine (89.5 g). ]H NMR (500 MHz, CDC13) δ 4.64 (s, 2H), 6.86-6.90 (m, 1H), 7.47-7.52 (m, 2H), 7.60-7.65 (m, 1H), 7.82-7.88 (m, 1H), 7.98-8.03 (m, 2H).
6-chloro-l-(4-methoxybenzyl)-3-methylpyrimidine-2,4(lH,3H)-dione

The mixture of 6-chloro-3-methyluracil (100 g), p-methoxybenzylchloride (107 g), K2CO3 (86.1 g) and DMAc (600 mL) is stirred at 75°C for 4 h. Water (400 mL) is added at 45°C and the mixture is cooled to room temperature. Water (800 mL) is added and the mixture is stirred at room temperature. The crystals are isolated by filtration, washed with the mixture of DMAc and water (1:2, 200mL) and dried to give 6-chloro-l-(4-methoxybenzyl)-3-methylpyrimidine-2,4(lH,3H)-dione (167 g). ]H NMR (500 MHz, CDC13) δ 3.35 (s, 3H), 3.80 (s, 3H), 5.21 (s, 2H), 5.93 (s, 1H), 6.85-6.89 (m, 2H), 7.26-7.32 (m, 2H).
izinyl-l-(4-methoxybenzyl)-3-methylpyrimidine-2,4(lH,3H)-dione

The mixture of 6-chloro-l-(4-methoxybenzyl)-3-methylpyrimidine-2,4(lH,3H)-dione (165 g), IPA (990 mL), water (124 mL) and hydrazine hydrate (62.9 mL) is stirred at room temperature for 1 h. The mixture is warmed to 60°C and stirred at the same temperature for 4 h. Isopropyl acetate (1485 mL) is added at 45°C and the mixture is stirred at the same temperature for 0.5 h. The mixture is cooled at 10°C and stirred for lh. The crystals are isolated by filtration, washed with the mixture of IPA and isopropyl acetate (1:2, 330 mL) and dried to give 6-hydrazinyl-l-(4-methoxybenzyl)-3-methylpyrimidine-2,4(lH,3H)-dione (153 g). ]H NMR (500 MHz, DMSO-i¾) δ 3.12 (s, 3H), 3.71 (s, 3H), 4.36 (s, 2H), 5.01 (s, 2H), 5.14 (s, 1H), 6.87-6.89 (m, 2H), 7.12-7.17 (m, 2H), 8.04 (s, 1H).
7-(4-methoxybenzyl)-5-methyl-2H-pyrazolo[3,4-d]pyrimidine-4,6(5H,7H)-dione

To the mixture of DMF (725 mL) and 6-hydrazinyl-l-(4-methoxybenzyl)-3-methylpyrimidine-2,4(lH,3H)-dione (145 g) is added POCI3 (58.5 mL) at 5°C. The mixture is stirred at room temperature for 1 h. Water (725 mL) is added at 50°C and the mixture is stirred at room temperature for 1 h. The crystals are isolated by filtration, washed with the mixture of DMF and water (1:1, 290 mL) and dried to give 7-(4-methoxybenzyl)-5-methyl-
2H-pyrazolo[3,4-d]pyrimidine-4,6(5H,7H)-dione (145 g). ]H NMR (500 MHz, DMSO-i¾) δ 3.23 (s, 3H), 3.71 (s, 3H), 5.05 (s, 2H), 6.82-6.90 (m, 2H), 7.28-7.36 (m, 2H), 8.48 (s, IH), 13.51 (br, IH).
2-(4-(6-fluoropyridin-2-yl)benzyl)-7-(4-methoxybenzyl)-5-methyl-2H-pyrazolo[3,4-d]pyrimidine-4,6(5H,7H)-dione

The mixture of 2-(4-(chloromethyl)phenyl)-6-fluoropyridine (100 g), 7-(4-methoxybenzyl)-5-methyl-2H-pyrazolo[3,4-d]pyrimidine-4,6(5H,7H)-dione (129 g), K2CO3(62.3 g) and DMAc (1500 mL) is stirred at 45°C for 5 h. Water (1500 mL) is added at 40°C and the mixture is stirred at room temperature for 1 h. The crystals are isolated by filtration, washed with the mixture of DMAc and water (1:1, 500 mL) and dried to give 2-(4-(6-fluoropyridin-2-yl)benzyl)-7-(4-methoxybenzyl)-5-methyl-2H-pyrazolo[3,4-d]pyrimidine-4,6(5H,7H)-dione (207 g). ]H NMR (500 MHz, DMSO- ) δ 3.21 (s, 3H), 3.66 (s, 3H), 4.98 (s, 2H), 5.45 (s, 2H), 6.77-6.82 (m, 2H), 7.13-7.16 (m, IH), 7.25-7.30 (m, 2H), 7.41-7.44 (m, 2H), 7.92-7.96 (m, IH), 8.04-8.11 (m, 3H), 8.68 (s, IH).
2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-2H-pyrazolo[3,4-d]pyrimidine-4,6(5H,7H)-dione

The mixture of 2-(4-(6-fluoropyridin-2-yl)benzyl)-7-(4-methoxybenzyl)-5-methyl-2H-pyrazolo[3,4-d]pyrimidine-4,6(5H,7H)-dione (105 g), CF3COOH (300 mL) and
CF3SO3H (100 g) is stirred at room temperature for 10 h. Acetonitrile (1000 mL) is added. The mixture is added to the mixture of 25% N¾ (1000 mL) and acetonitrile (500 mL) at 10°C. The mixture is stirred at room temperature for 1 h. The crystals are isolated by filtration, washed with the mixture of acetonitirile and water (1:1, 500 mL) and dried to give the crude product. The mixture of the crude product and AcOEt (1200 mL) is stirred at room temperature for 1 h. The crystals are isolated by filtration, washed with AcOEt (250 mL) and dried to give 2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-2H-pyrazolo[3,4-d]pyrimidine-4,6(5H,7H)-dione (75.3 g). ]H NMR (500 MHz, DMSO-rf6) δ 3.16 (s, 3H), 3.50-4.00 (br, 1H), 5.40 (s, 2H), 7.13-7.16 (m, 1H), 7.41-7.44 (m, 2H), 7.91-7.94 (m, 1H), 8.04-8.10 (m, 3H), 8.60 (s, 1H).
2-(4-(6-fluoropyridin-2-yl)benzyl)-6-(((lR,2R)-2-hydroxycyclopentyl)amino)-5-methyl-2H-pyrazolo[3,4-d]pyrimidin-4(5H)-one

The mixture of BOP reagent (126 g), 2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-2H-pyrazolo[3,4-d]pyrimidine-4,6(5H,7H)-dione (80 g), DBU (136 mL) and THF (1120 mL) is stirred at room temperature for 1 h. (lR,2R)-2-Aminocyclopentanol hydrochloride (37.6 g) and THF (80 mL) are added and the mixture is stirred at room temperature for 5 h. After the addition of 5% NaCl (400 mL) and AcOEt (800 mL), the organic layer is separated. The organic layer is washed with 10% NaCl (400 mL), 1M HC1 15% NaCl (400 mL), 5% NaCl (400 mL), 5% NaHC03 (400 mL) and 5%NaCl (400 mL) successively. After treatment with active charcoal, the organic layer is concentrated to 400 mL. After the addition of acetonitrile (800 mL), the mixture is concentrated to 400 mL. After the addition of acetonitrile (800 mL), seed crystals are added at 40°C. The mixture is concentrated to 400 mL. Water (800 mL) is added at room temperature and the mixture is stirred for 2 h. The crystals are isolated by filtration, washed with the mixture of acetonitrile and water (1:2, 400 mL) and dried to give 2-(4-(6-fluoropyridin-2-yl)benzyl)-6-(((lR,2R)-2-
hydroxycyclopentyl)amino)-5-methyl-2H-pyrazolo[3,4-d]pyrimidin-4(5H)-one (81.7 g). ]H NMR (500 MHz, CDC13) δ 1.47-1.59 (m, 1H), 1.68-1.93 (m, 3H), 2.02-2.12 (m, 1H), 2.24-2.34 (m, 1H), 3.42 (s, 3H), 3.98-4.12 (m, 2H), 4.68-4.70 (m, 1H), 5.37 (s, 2H), 6.86-6.90 (m, 1H), 7.36-7.42 (m, 2H), 7.58-7.63 (m, 1H), 7.81-7.88 (m, 1H), 7.89 (s, 1H), 7.97-8.01 (m, 2H).
(6aR,9aS)-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-5,6a,7,8,9,9a-hexahydrocyclopenta[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one

The mixture of 2-(4-(6-fluoropyridin-2-yl)benzyl)-6-(((lR,2R)-2-hydroxycyclopentyl)amino)-5-methyl-2H-pyrazolo[3,4-d]pyrimidin-4(5H)-one (80 g), p-toluenesulfonylchloride (38.6 g), Et3N (28.2 mL), N,N-dimethylaminopyridine (24.7 g) and THF (800 mL) is stirred at 50°C for 10 h. To the mixture is added 8M NaOH (11.5 mL) at room temperature and the mixture is stirred for 2 h. After the addition of 5% NaCl (400 mL) and AcOEt (800 mL), the organic layer is separated. The organic layer is washed with 5 NaCl (400 mL) twice. The organic layer is concentrated to 240 mL. After the addition of MeOH (800 mL), the mixture is concentrated to 240 mL. After the addition of MeOH (800 mL), the mixture is concentrated to 240 mL. After the addition of MeOH (160 mL), the mixture is stirred at room temperature for 1 h and at 0°C for 1 h. The crystals are isolated by filtration, washed with cold MeOH (160 mL) and dried to give (6aR,9aS)-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-5,6a,7,8,9,9a-hexahydrocyclopenta[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one (55.7 g). ]H NMR (500 MHz, CDC13) δ 1.39-1.54 (m, 1H), 1.58-1.81 (m, 3H), 1.81-1.92 (m, 1H), 2.12-2.22 (m, 1H), 3.28 (s, 3H), 4.61-4.70 (m, 2H), 5.20 (s, 2H), 6.79-6.85 (m, 1H), 7.25-7.32 (m, 2H), 7.53-7.58 (m, 1H), 7.68 (s, 1H), 7.75-7.83 (m, 1H), 7.92-7.98 (m, 2H).
(6aR,9aS)-3-chloro-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-5,6a,7,8,9,9a-
hexahydrocyclopenta[4,5]imi ]pyrimidin-4(2H)-one

The mixture of (6aR,9aS)-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-5,6a,7,8,9,9a-hexahydrocyclopenta[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one (50 g) and toluene (1000 mL) is concentrated to 750 mL under the nitrogen atmosphere. Toluene (250 mL) and NCS (24 g) is added. To the mixture is added LiHMDS (1M THF solution, 204 mL) at 0°C and the mixture is stirred for 0.5 h. To the mixture is added 20% NH4C1 (50 mL) at 5°C. The mixture is concentrated to 250 mL. After the addition of EtOH (250 mL), the mixture is concentrated to 150 mL. After the addition of EtOH (250 mL), the mixture is concentrated to 200 mL. After the addition of EtOH (200 mL), the mixture is warmed to 50°C. Water (300 mL) is added and the mixture is stirred at 50°C for 0.5 h. After stirring at room temperature for 1 h, the crystals are isolated by filtration, washed with the mixture of EtOH and water (1:1, 150 mL) and dried to give (6aR,9aS)-3-chloro-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-5,6a,7,8,9,9a-hexahydrocyclopenta[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one (51.1 g). ]H NMR (500 MHz, CDC13) δ 1.46-1.61 (m, 1H), 1.67-1.90 (m, 3H), 1.92-2.00 (m, 1H), 2.19-2.27 (m, 1H), 3.37 (s, 3H), 4.66-4.77 (m, 2H), 5.34 (s, 2H), 6.87-6.93 (m, 1H), 7.35-7.41 (m, 2H), 7.59-7.65 (m, 1H), 7.82-7.91 (m, 1H), 7.97-8.05 (m, 2H).
EXAMPLE 1
Crystals of (6a/f,9a5)-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one free base mono-ethanol solvate

The mixture of (6a/?,9a5')-3-chloro-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-5,6a,7,8,9,9a-hexahydrocyclopenta[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one (2.5 g), K2C03 (1.53 g), Pd(OAc)2 (12.5 mg), Xantphos (32 mg), aniline (0.76 mL), and xylene (12.5 mL) is stirred at 125°C for 7 h under nitrogen atmosphere. After addition of water (12.5 mL), the organic layer is separated. The organic layer is washed with water (12.5 mL) twice. The organic layer is extracted with the mixture of DMAc (6.25 mL) and 0.5N HCl (12.5 mL). The organic layer is extracted with the mixture of DMAc (3.2 mL) and 0.5N HCl (6.25 mL). After addition of DMAc (6.25 mL), xylene (12.5 mL) and 25 wt % aqueous NH3 solution to the combined aqueous layer, the organic layer is separated. The aqueous layer is extracted with xylene (6.25 mL). The combined organic layer is washed with water (12.5 mL), 2.5 wt % aqueous 1 ,2-cyclohexanediamine solution (12.5 mL) twice and water (12.5 mL) successively. After treatment with active charcoal, the organic layer is concentrated. After addition of EtOH (12.5 mL), the mixture is concentrated. After addition of EtOH (12.5 mL), the mixture is concentrated. After addition of EtOH (12.5 mL), n-heptane (25 mL) is added at 70°C. The mixture is cooled to 5°C and stirred at same temperature. The crystals are isolated by filtration and dried to give (ea^^a^-S^a ^^^a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one free base mono-ethanol solvate (2.56 g) as crystals.
]H NMR (500 MHz, DMSO-d6) δ 0.98-1.13 (m, 3H), 1.34-1.52 (m, 1H), 1.54-1.83 (m, 4H), 2.03-2.17 (m, 1H), 3.11 (s, 3H), 3.39-3.54 (m, 2H), 4.29-4.43 (m, 1H), 4.51-4.60 (m, 1H), 4.60-4.70 (m, 1H), 5.15-5.35 (m, 2H), 6.71-6.88 (m, 3H), 7.05-7.29 (m, 5H), 7.81-7.93 (m, 1H), 7.94-8.11 (m, 3H), 8.67 (s, 1H).
EXAMPLE 4
Crystals of (6a/f,9a5)-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one free

Crystals of (6a«,9a5')-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one free base mono-n-propanol solvate (2.0 g) is dissolved with ethanol (10 mL) at 70°C. Isopropyl ether (20 mL) is added and the mixture is cooled to 45°C. Isopropyl ether (10 mL) is added and the mixture is stirred at 40°C. The mixture is cooled to 5°C and stirred at same temperature. The crystals are isolated by filtration and dried to give (ea/^^a^)-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one free base non-solvate (1.7 g) as crystals.
[0082] ]H NMR (500 MHz, DMSO-d6) δ 1.32-1.51 (m, 1H), 1.53-1.83 (m, 4H), 1.97-2.20 (m, 1H), 3.11 (s, 3H), 4.49-4.60 (m, 1H), 4.60-4.69 (m, 1H), 5.13-5.37 (m, 2H), 6.70-6.90 (m, 3H), 7.04-7.31 (m, 5H), 7.82-7.93 (m, 1H), 7.93-8.12 (m, 3H), 8.67 (s, 1H).
EXAMPLE 5
Crystals of (6a/f,9a5)-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one free base non-solvate

The mixture of (6a/?,9a5')-3-chloro-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-5,6a,7,8,9,9a-hexahydrocyclopenta[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one (25 g), K2C03 (15.4 g), Pd(OAc)2 (125 mg), Xantphos (321 mg), aniline (7.6 mL), DMAc (6.25 mL) and xylene (125 mL) is stirred at 125°C for 6.5 h under nitrogen atmosphere. After addition of water (125 mL) and DMAc (50 mL), the organic layer is separated. The organic layer is washed with the mixture of DMAc (50 mL) and water (125 mL) twice. The organic layer is extracted with the mixture of DMAc (50 mL) and 0.5N HCl (125 mL). The organic layer is extracted with the mixture of DMAc (50 mL) and 0.5N HCl (62.5 mL). After addition of DMAc (50 mL), xylene (125 mL) and 25 wt % aqueous NH3 solution (25 mL) to the combined aqueous layer, the organic layer is separated. The aqueous layer is extracted with xylene (62.5 mL). The combined organic layer is washed with the mixture of DMAc (50 mL) and water (125 mL), the mixture of DMAc (50 mL) and 2.5 wt % aqueous 1,2-cyclohexanediamine solution (125 mL) twice and the mixture of DMAc (50 mL) and water (125 mL) successively. After treatment with active charcoal (1.25 g), the organic layer is concentrated to 75 mL. After addition of EtOH (125 mL), the mixture is concentrated to 75 mL. After addition of EtOH (125 mL), the mixture is concentrated to 75 mL. After addition of EtOH (125 mL), n-heptane (250 mL) is added at 70°C. After addition of seed crystals of (6a/?,9a5')-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one non-solvate, the mixture is cooled to room temperature and stirred at room temperature. The crystals are isolated by filtration and dried to give (ea^^a^-S^a ^^^a-hexahydro-S-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo-[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one free base non-solvate (23.8 g) as crystals.
EXAMPLE 8
(6a/f,9a5)-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one mono-phosphate salt

[0094] Crystals of (6a«,9a5')-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one free base non-solvate (20 g) are dissolved in acetonitrile (60 mL) at 50°C. After addition of the active charcoal (1 g), the mixture is stirred at same temperature for 0.5 h. The active charcoal is removed by filtration and washed with acetonitrile (40 mL). The filtrate and the washing are combined and warmed to 50°C. A solution of 85 wt. % phosphoric acid (2.64 mL) in acetonitrile (100 mL) is added. After addition of water (20 mL), the mixture is stirred at 50°C for lh. The crystals are isolated by filtration, washed with acetonitrile (60 mL x 3) and dried to give (6a/?,9a5')-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one mono-phosphate salt (20.5 g).
EXAMPLE 9
(6a/f,9a5)-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one mono-phosphate salt

[0095] Crystals of (6a«,9a5')-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one free base mono-ethanol solvate (4 g) are dissolved in acetonitrile (12 mL) at 50°C. After addition of active charcoal (0.2 g), the mixture is stirred at same temperature for 0.5 h. Active charcoal is removed by filtration and washed with acetonitrile (8 mL). The filtrate and the washing are combined and warmed to 50°C. A solution of 85 wt. % phosphoric acid (0.528 mL) in acetonitrile (20 mL) is added. After addition of water (4 mL), the mixture is stirred at 50°C for lh. The crystals are isolated by filtration, washed with acetonitrile (12 mL x 3) and dried to give (6a/?,9a5')-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one mono-phosphate salt (4.01 g).
EXAMPLE 10
(6a/f,9a5)-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one mono-phosphate salt

Crystals of (6a«,9a5')-5,6a,7,8,9,9a-Hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one free base non-solvate (20 g) are dissolved in acetone (60 mL) at 32°C. After addition of active charcoal (1 g), the mixture is stirred at same temperature for 0.5 h. Active charcoal is removed by filtration and washed with acetone (40 mL). The filtrate and the washing are combined and warmed to 39°C. A solution of 85 wt. % phosphoric acid (2.64 mL) in acetone (100 mL) is added. After addition of water (20 mL), the mixture is stirred at 40°C for lh. The crystals are isolated by filtration, washed with acetone (60 mL x 3) and dried to give (6a/?,9a5')-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one mono-phosphate salt (22.86 g).
EXAMPLE 11
(6a/f,9a5)-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one mono-phosphate salt

Crystals of (6a«,9a5')-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one free base mono-ethanol solvate (20 g) are dissolved in acetone (60 mL) at 38°C. After addition of active charcoal (1 g), the mixture is stirred at same temperature for 0.5 h. Active charcoal is removed by filtration and washed with acetone (40 mL). The filtrate and the washing are combined and warmed to 38°C. A solution of 85 wt. % phosphoric acid (2.64 mL) in acetone (100 mL) is added. After addition of water (20 mL), the mixture is stirred at 40°C for lh. The crystals are isolated by filtration, washed with acetone (60 mL x 3) and dried to give (6a/?,9a5')-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-2-((4-(6-fluoropyridin-2-yl)phenyl)methyl)-cyclopent[4,5]imidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one mono-phosphate salt (23.2 g).
PAPER
A diverse set of 3-aminopyrazolo[3,4-d]pyrimidinones was designed and synthesized. The structure–activity relationships of these polycyclic compounds as phosphodiesterase 1 (PDE1) inhibitors were studied along with their physicochemical and pharmacokinetic properties. Systematic optimizations of this novel scaffold culminated in the identification of a clinical candidate, (6aR,9aS)-2-(4-(6-fluoropyridin-2-yl)benzyl)-5-methyl-3-(phenylamino)-5,6a,7,8,9,9a-hexahydrocyclopenta[4,5]imidazo[1,2-a]pyrazolo[4,3-e]pyrimidin-4-(2H)-one phosphate (ITI-214), which exhibited picomolar inhibitory potency for PDE1, demonstrated excellent selectivity against all other PDE families and showed good efficacy in vivo. Currently, this investigational new drug is in Phase I clinical development and being considered for the treatment of several indications including cognitive deficits associated with schizophrenia and Alzheimer’s disease, movement disorders, attention deficit and hyperactivity disorders, and other central nervous system (CNS) and non-CNS disorders
Discovery of Potent and Selective Inhibitors of Phosphodiesterase 1 for the Treatment of Cognitive Impairment Associated with Neurodegenerative and Neuropsychiatric Diseases
†Intra-Cellular Therapies, Inc., 430 East 29th Street, Suite 900, New York, New York 10016, United States
‡ Department of Structural Biology, Takeda California, Inc., 10410 Science Center Drive, San Diego, California 92121,United States
§ Pharmaceutical Research Division, Takeda Pharmaceutical Company, Ltd., 26-1, Muraoka-Higashi 2-Chome, Fujisawa, Kanagawa 251-8555, Japan
∥ Department of Neurosciences, University of California, San Diego, 9500 Gilman Drive, #0608, La Jolla, California 92093,United States
J. Med. Chem., 2016, 59 (3), pp 1149–1164
DOI: 10.1021/acs.jmedchem.5b01751
Publication Date (Web): January 20, 2016
Copyright © 2016 American Chemical Society
*Phone: 646-440-9388. E-mail: pli@intracellulartherapies.com.
Step g. (6aR,9aS)-5-Methyl-3-(phenylamino)-2-(4-(6-fluoropyridin-2-yl)-benzyl)-5,6a,7,8,9,9a-hexahydrocyclopent[4,5]imidazo[1,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one phosphate (3)
............ to give (6aR,9aS)-5-methyl-3-(phenylamino)-2-(4-(6-fluoropyridin-2-yl)-benzyl)-5,6a,7,8,9,9a-hexahydrocyclopent[4,5]imidazo[1,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one as an off-white solid
BASE FORM
1H NMR (500 MHz, CDCl3) δ 7.89 (d, J = 8.3 Hz, 2H), 7.86–7.79 (m, 1H), 7.58 (dd, J = 7.6, 2.5 Hz, 1H), 7.35–7.26 (m, 2H), 7.15–7.08 (m, 1H), 7.05 (d, J = 8.3 Hz, 2H), 6.94 (d, J = 7.6 Hz, 2H), 6.90 (br, 1H), 6.86 (dd, J = 8.1, 3.0 Hz, 1H), 4.96 (s, 2H), 4.88–4.70 (m, 2H), 3.38 (s, 3H), 2.29 (dd, J = 13.0, 6.1 Hz, 1H), 2.15–1.96 (m, 1H), 1.90–1.71 (m, 3H), 1.65–1.52 (m, 1H).
13C NMR (126 MHz, CDCl3) δ 163.4 (d, JCF = 239 Hz), 159.7, 155.7 (d, JCF = 13 Hz), 153.0, 147.6, 144.1, 141.7 (d, JCF = 8 Hz), 140.5, 137.3, 137.1, 129.6, 127.8, 127.1, 124.1, 120.2, 117.3 (d, JCF = 4 Hz), 107.9 (d, JCF = 38 Hz), 89.5, 69.9, 62.6, 52.8, 35.4, 32.3, 28.5, 23.2.
MS (ESI) m/z 508.3 [M + H]+.
PHOSPHATE SALT
1H NMR (500 MHz, DMSO-d6) δ 8.71 (br, 1H), 8.10–8.01 (m, 1H), 7.98 (d, J = 8.3 Hz, 2H), 7.89 (dd, J = 7.6, 2.6 Hz, 1H), 7.23 (d, J = 8.4 Hz, 2H), 7.16 (dd, J = 8.5, 7.3 Hz, 2H), 7.12 (dd, J = 8.1, 2.8 Hz, 1H), 6.86–6.81 (m, 1H), 6.80–6.76 (m, 2H), 5.34–5.19 (m, 2H), 4.77–4.64 (m, 1H), 4.62–4.53 (m, 1H), 3.12 (s, 3H), 2.11 (dd, J = 13.4, 5.7 Hz, 1H), 1.81–1.57 (m, 4H), 1.54–1.41 (m, 1H).
13C NMR (126 MHz, CDCl3) δ 162.6 (d, JCF = 236 Hz), 155.9, 154.4 (d, JCF= 13 Hz), 152.4, 146.6, 143.0 (d, JCF = 8 Hz), 142.5, 141.8, 138.1, 136.0, 128.7, 127.5, 126.7, 120.4, 117.7 (d, JCF = 4 Hz), 116.0, 108.1 (d, JCF = 37 Hz), 90.3, 66.3, 62.4, 50.6, 34.2, 31.2, 28.5, 22.5.
MS (ESI) m/z 508.3 [M + H]+.
HRMS (ESI) m/z calcd for C29H27N7OF [M (free base)+H]+, 508.2261; found, 508.2272.
HPLC purity, 100.0%; retention time, 13.0 min.
PATENT
The synthetic methods disclosed in WO 2009/075784 and WO 2013/192556 are particularly applicable, as they include the methods to prepare the compound of Formula I-B. Those skilled in the art will readily see how those methods are applicable to the synthesis of the compounds of the present invention.

Formula I-B
For example, Compounds of the Invention wherein any one or more of R1 through R8 are D, can be prepared from the corresponding aminocyclopentanol, according to the method described in WO 2009/075784 or WO 2013/192556. For example, by reacting said aminocyclopentanol, optionally as its acid salt, with Intermediate A in the presence of a coupling agent, e.g., benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate (BOP reagent), and a base, e.g., l,8-diazabicyclo[5.4.0]undec-7-ene (DBU), in a solvent such as tetrahydrofuran (THF). The intermediate alcohol is then cyclized by treatment with toluenesulfonyl chloride (TsCl) in the presence of one or more bases, such as dimethylaminopyridine (DMAP) and triethylamine (TEA) in a solvent, such as THF. The reaction is summarized in the following scheme:

The required aminocyclopentanols can be prepared by methods known to those skilled in the art. For example, the aminocyclopentanol wherein R1 is D can be prepared via a reductive amination procedure that uses a reducing agent such as sodium triacetoxyborodeuteride or sodium borodeuteride as the reducing agent. For example, an optionally protected (R)-2-hydroxycyclopentanone can be reacted with 4-methoxybenzylamine in the presence of sodium triacetoxyborodeuteride to yield the desired deuterated secondary amine, wherein P is the protecting group. Reaction of the resulting amine with a strong acid such as trifluoromethanesulfonic acid (TMFSA) will result in removal of the 4-methoxybenzyl group and the protecting group to yield the desired aminocyclopentanol. Those skilled in the art will know how to choose a suitable protecting group for the secondary alcohol such that deprotection can take place during the acid treatment step (e.g., a tert-butyldimethylsilyl group or a tert-butoxycarbonyl group). Alternatively, those skilled in the art could choose a protecting group that would survive this step. If desired, the protected intermediate can be purified by chiral HPLC in order to enhance the optical purity of the final

As another example, Compounds of the Invention wherein any one or more of R9 to R15 or R21 to R22 are D can be prepared from the corresponding benzyl halide, according to the method described in WO 2009/075784 or WO 2013/192556. For example, by reacting said benzyl halide with the Intermediate B in the presence of suitable base, such as cesium carbonate or potassium carbonate, in a suitable solvent, such as dimethylformamide or dimethylacetamide. The corresponding benzyl halide can be prepared by methods well known to those skilled in the art. The reaction is summarized in the following scheme:

As another example, compounds of the invention wherein any one or more of R16 to R20 are D can be prepared from the corresponding phenyl
isothiocyanate, according to the method described in WO 2009/075784 or WO
2013/192556. For example, by reacting said phenyl isothiocyanate with Intermediate C in a suitable solvent, such as dimethylformamide. The corresponding phenyl isothiocyanate can be prepared by methods well known to those skilled in the art. The reaction is summarized in the following scheme:

Alternatively, compounds of the invention wherein any one or more of R16 to R20 are D can be prepared from the corresponding aniline, according to the method described in WO 2009/075784 or WO 2013/192556. For example, by reacting said aniline with Intermediate D and a strong base, such as lithium
hexamethyldisilylazide (LiHMDS), in a suitable solvent, such as THF at elevated temperature. Such a reaction can also be achieved by catalytic amination using a catalyst, such as tris(dibenzylideneacetone)dipalladium (Pd2(dba)3), and a ligand, such as Xantphos. The corresponding aniline can be prepared by methods well known to those skil


Formula I-B
For example, Compounds of the Invention wherein any one or more of R1 through R8 are D, can be prepared from the corresponding aminocyclopentanol, according to the method described in WO 2009/075784 or WO 2013/192556. For example, by reacting said aminocyclopentanol, optionally as its acid salt, with Intermediate A in the presence of a coupling agent, e.g., benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate (BOP reagent), and a base, e.g., l,8-diazabicyclo[5.4.0]undec-7-ene (DBU), in a solvent such as tetrahydrofuran (THF). The intermediate alcohol is then cyclized by treatment with toluenesulfonyl chloride (TsCl) in the presence of one or more bases, such as dimethylaminopyridine (DMAP) and triethylamine (TEA) in a solvent, such as THF. The reaction is summarized in the following scheme:

The required aminocyclopentanols can be prepared by methods known to those skilled in the art. For example, the aminocyclopentanol wherein R1 is D can be prepared via a reductive amination procedure that uses a reducing agent such as sodium triacetoxyborodeuteride or sodium borodeuteride as the reducing agent. For example, an optionally protected (R)-2-hydroxycyclopentanone can be reacted with 4-methoxybenzylamine in the presence of sodium triacetoxyborodeuteride to yield the desired deuterated secondary amine, wherein P is the protecting group. Reaction of the resulting amine with a strong acid such as trifluoromethanesulfonic acid (TMFSA) will result in removal of the 4-methoxybenzyl group and the protecting group to yield the desired aminocyclopentanol. Those skilled in the art will know how to choose a suitable protecting group for the secondary alcohol such that deprotection can take place during the acid treatment step (e.g., a tert-butyldimethylsilyl group or a tert-butoxycarbonyl group). Alternatively, those skilled in the art could choose a protecting group that would survive this step. If desired, the protected intermediate can be purified by chiral HPLC in order to enhance the optical purity of the final

As another example, Compounds of the Invention wherein any one or more of R9 to R15 or R21 to R22 are D can be prepared from the corresponding benzyl halide, according to the method described in WO 2009/075784 or WO 2013/192556. For example, by reacting said benzyl halide with the Intermediate B in the presence of suitable base, such as cesium carbonate or potassium carbonate, in a suitable solvent, such as dimethylformamide or dimethylacetamide. The corresponding benzyl halide can be prepared by methods well known to those skilled in the art. The reaction is summarized in the following scheme:

As another example, compounds of the invention wherein any one or more of R16 to R20 are D can be prepared from the corresponding phenyl
isothiocyanate, according to the method described in WO 2009/075784 or WO
2013/192556. For example, by reacting said phenyl isothiocyanate with Intermediate C in a suitable solvent, such as dimethylformamide. The corresponding phenyl isothiocyanate can be prepared by methods well known to those skilled in the art. The reaction is summarized in the following scheme:

Alternatively, compounds of the invention wherein any one or more of R16 to R20 are D can be prepared from the corresponding aniline, according to the method described in WO 2009/075784 or WO 2013/192556. For example, by reacting said aniline with Intermediate D and a strong base, such as lithium
hexamethyldisilylazide (LiHMDS), in a suitable solvent, such as THF at elevated temperature. Such a reaction can also be achieved by catalytic amination using a catalyst, such as tris(dibenzylideneacetone)dipalladium (Pd2(dba)3), and a ligand, such as Xantphos. The corresponding aniline can be prepared by methods well known to those skil

EXAMPLE 1. (6aR,9a5)-5-Methyl-3-(2,3,4,5,6-pentadeuterophenylamino)-2-(4-(6-fluoropyridin-2-yl)-benzyl)-5,6fl,7,8,9,9fl-hexahydrocyclopent[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one

To a solution of (6a/?,9a5')-5,6a,7,8,9,9a-hexahydro-3-chloro-5-methyl-2-(4-(6-fluoropyridin-2-yl)-benzyl)-cyclopent[4,5]irnidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one (200 mg, 0.444 mmol) and 2,3,4,5,6-pentadeuteroaniline (162 μΐ,, 1.8 mmol) in anhydrous 2-methyltetrahydrofuran (3 mL) is added LiHMDS (1.0 M in THF, 0.89 mL) dropwise at room temperature under argon atmosphere. The reaction mixture is gradually heated to 75 °C over a period of 90 min, and then heated at 75 °C for an hour. The mixture is cooled with an ice bath and then quenched by adding 0.2 mL of water. After solvent evaporation, the residue is dissolved in DMF and then filter with a 0.45 m microfilter. The collected filtrated is purified with a semi-preparative HPLC system using a gradient of 0 - 70% acetonitrile in water containing 0.1% formic acid over 16 min to give (6a/?,9a5')-5-methyl-3-(2,3,4,5,6-pentadeuterophenylamino)-2-(4-(6-fluoropyridin-2-yl)-benzyl)-5,6fl,7,8,9,9fl-hexahydrocyclopent[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one as a formate salt, which is dissolved in ethyl acetate, basified with 12.5 mL of 5% sodium carbonate, and then extracted with ethyl acetate three times. The combined organic phase is evaporated to dryness. The residue is dissolved in 4.5 mL of THF and then filter through a 0.45 m microfilter. The filtrate is evaporated to dryness and further dried under vacuum to give (6a/?,9a5')-5-methyl-3-(2,3,4,5,6-pentadeuterophenylamino)-2-(4-(6-fluoropyridin-2-yl)-benzyl)-5,6fl,7,8,9,9fl-hexahydrocyclopent[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one as a white solid (185.8 mg, 81.6% yield). ¾ NMR (400 MHz, CDCb) δ 7.88 (d, / = 8.4 Hz, 2H), 7.88 - 7.77 (m, 1H), 7.58 (dd, J = 7.5, 2.4 Hz, 1H), 7.05 (d, J = 8.3 Hz, 2H), 6.90 - 6.80 (m, 2H), 4.94 (s, 2H), 4.82 - 4.68 (m, 2H), 3.34 (s, 3H), 2.27 (dd, / = 12.4, 5.7 Hz, 1H), 2.09 - 1.91 (m, 1H), 1.91 - 1.67 (m, 3H), 1.67 - 1.49 (m, 1H).MS (ESI) m/z 513.3 [M+H]+.

To a solution of (6a/?,9a5')-5,6a,7,8,9,9a-hexahydro-3-chloro-5-methyl-2-(4-(6-fluoropyridin-2-yl)-benzyl)-cyclopent[4,5]irnidazo[l,2-fl]pyrazolo[4,3-e]pyrimidin-4(2H)-one (200 mg, 0.444 mmol) and 2,3,4,5,6-pentadeuteroaniline (162 μΐ,, 1.8 mmol) in anhydrous 2-methyltetrahydrofuran (3 mL) is added LiHMDS (1.0 M in THF, 0.89 mL) dropwise at room temperature under argon atmosphere. The reaction mixture is gradually heated to 75 °C over a period of 90 min, and then heated at 75 °C for an hour. The mixture is cooled with an ice bath and then quenched by adding 0.2 mL of water. After solvent evaporation, the residue is dissolved in DMF and then filter with a 0.45 m microfilter. The collected filtrated is purified with a semi-preparative HPLC system using a gradient of 0 - 70% acetonitrile in water containing 0.1% formic acid over 16 min to give (6a/?,9a5')-5-methyl-3-(2,3,4,5,6-pentadeuterophenylamino)-2-(4-(6-fluoropyridin-2-yl)-benzyl)-5,6fl,7,8,9,9fl-hexahydrocyclopent[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one as a formate salt, which is dissolved in ethyl acetate, basified with 12.5 mL of 5% sodium carbonate, and then extracted with ethyl acetate three times. The combined organic phase is evaporated to dryness. The residue is dissolved in 4.5 mL of THF and then filter through a 0.45 m microfilter. The filtrate is evaporated to dryness and further dried under vacuum to give (6a/?,9a5')-5-methyl-3-(2,3,4,5,6-pentadeuterophenylamino)-2-(4-(6-fluoropyridin-2-yl)-benzyl)-5,6fl,7,8,9,9fl-hexahydrocyclopent[4,5]imidazo[l,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one as a white solid (185.8 mg, 81.6% yield). ¾ NMR (400 MHz, CDCb) δ 7.88 (d, / = 8.4 Hz, 2H), 7.88 - 7.77 (m, 1H), 7.58 (dd, J = 7.5, 2.4 Hz, 1H), 7.05 (d, J = 8.3 Hz, 2H), 6.90 - 6.80 (m, 2H), 4.94 (s, 2H), 4.82 - 4.68 (m, 2H), 3.34 (s, 3H), 2.27 (dd, / = 12.4, 5.7 Hz, 1H), 2.09 - 1.91 (m, 1H), 1.91 - 1.67 (m, 3H), 1.67 - 1.49 (m, 1H).MS (ESI) m/z 513.3 [M+H]+.
Intra-Cellular Therapies and Takeda Announce Mutual Termination of Collaboration to Develop Phosphodiesterase (PDE1) Inhibitors for CNS Disorders
NEW YORK and OSAKA, Japan, Nov. 3, 2014 (GLOBE NEWSWIRE) -- Intra-Cellular Therapies, Inc. (Nasdaq:ITCI) and Takeda Pharmaceutical Company Limited announced today that they have entered into an agreement to mutually terminate the February 2011 license agreement covering Intra-Cellular Therapies' proprietary compound ITI-214 and related PDE1 inhibitors and to return the rights for these compounds to Intra-Cellular Therapies.
Under the terms of the agreement, Intra-Cellular Therapies has regained all worldwide development and commercialization rights for the compounds previously licensed to Takeda. Takeda will be responsible for transitioning the compounds back toIntra-Cellular Therapies and will not participate in future development or commercialization activities. After transition of the program, Intra-Cellular Therapies plans to continue the clinical development of PDE1 inhibitors for the treatment of central nervous system, cardiovascular and other disorders.
"We are grateful for Takeda's substantial efforts in advancing this program into clinical development," said Dr. Sharon Mates, Chairman and CEO of Intra-Cellular Therapies. "This provides us with the opportunity to unify our PDE1 platform and we look forward to continuing the development of ITI-214 and our other PDE1 inhibitors."
Intra-Cellular Therapies will discuss the PDE1 program in its previously announced earnings call on Monday, November 3, 2014 at 8:30 a.m. Eastern Time. To participate in the conference call, please dial 844-835-6563 (U.S.) or 970-315-3916 (International) five to ten minutes prior to the start of the call. The participant passcode is 25568442.
About PDE1 Inhibitors
PDE1 inhibitors are unique, orally available, investigational drug candidates being developed for the treatment of cognitive impairments accompanying schizophrenia, Alzheimer's disease and other neuropsychiatric disorders and neurological diseases and may also treat patients with Attention Deficit Hyperactivity Disorder and Parkinson's disease. These compounds may also have the potential to improve motor dysfunction associated with these conditions and may also have the potential to treat patients with multiple sclerosis and other autoimmune diseases and pulmonary arterial hypertension. These compounds are very selective for the PDE1 subfamily relative to other PDE subfamilies. They have no known significant off target activities at other enzymes, receptors or ion channels.
About Intra-Cellular Therapies
Intra-Cellular Therapies, Inc. (the "Company") is developing novel drugs for the treatment of neuropsychiatric and neurodegenerative disease and other disorders of the central nervous system ("CNS"). The Company is developing its lead drug candidate, ITI-007, for the treatment of schizophrenia, behavioral disturbances in dementia, bipolar disorder and other neuropsychiatric and neurological disorders. The Company is also utilizing its phosphodiesterase platform and other proprietary chemistry platforms to develop drugs for the treatment of CNS disorders.
About Takeda Pharmaceutical Company Limited
Located in Osaka, Japan, Takeda is a research-based global company with its main focus on pharmaceuticals. As the largest pharmaceutical company in Japan and one of the global leaders of the industry, Takeda is committed to strive towards better health for people worldwide through leading innovation in medicine. Additional information about Takeda is available through its corporate website, www.Takeda.com.

Source: Intra-Cellular Therapies, Inc.; Takeda Pharmaceutical Company Limited
| ||||||
"We are grateful for Takeda's substantial efforts in advancing this program into clinical development," said Dr. Sharon Mates, Chairman and CEO of Intra-Cellular Therapies. "This provides us with the opportunity to unify our PDE1 platform and we look forward to continuing the development of ITI-214 and our other PDE1 inhibitors."
Intra-Cellular Therapies will discuss the PDE1 program in its previously announced earnings call on Monday, November 3, 2014 at 8:30 a.m. Eastern Time. To participate in the conference call, please dial 844-835-6563 (U.S.) or 970-315-3916 (International) five to ten minutes prior to the start of the call. The participant passcode is 25568442.
About PDE1 Inhibitors
PDE1 inhibitors are unique, orally available, investigational drug candidates being developed for the treatment of cognitive impairments accompanying schizophrenia, Alzheimer's disease and other neuropsychiatric disorders and neurological diseases and may also treat patients with Attention Deficit Hyperactivity Disorder and Parkinson's disease. These compounds may also have the potential to improve motor dysfunction associated with these conditions and may also have the potential to treat patients with multiple sclerosis and other autoimmune diseases and pulmonary arterial hypertension. These compounds are very selective for the PDE1 subfamily relative to other PDE subfamilies. They have no known significant off target activities at other enzymes, receptors or ion channels.
About Intra-Cellular Therapies
Intra-Cellular Therapies, Inc. (the "Company") is developing novel drugs for the treatment of neuropsychiatric and neurodegenerative disease and other disorders of the central nervous system ("CNS"). The Company is developing its lead drug candidate, ITI-007, for the treatment of schizophrenia, behavioral disturbances in dementia, bipolar disorder and other neuropsychiatric and neurological disorders. The Company is also utilizing its phosphodiesterase platform and other proprietary chemistry platforms to develop drugs for the treatment of CNS disorders.
About Takeda Pharmaceutical Company Limited
Located in Osaka, Japan, Takeda is a research-based global company with its main focus on pharmaceuticals. As the largest pharmaceutical company in Japan and one of the global leaders of the industry, Takeda is committed to strive towards better health for people worldwide through leading innovation in medicine. Additional information about Takeda is available through its corporate website, www.Takeda.com.
Source: Intra-Cellular Therapies, Inc.; Takeda Pharmaceutical Company Limited
| US20080188492 * | Jun 6, 2006 | Aug 7, 2008 | Intra-Cellular Therapies, Inc | Organic Compounds |
| US20100273754 * | Dec 6, 2008 | Oct 28, 2010 | Peng Li | Organic compounds |
| US20110237561 * | Dec 7, 2009 | Sep 29, 2011 | Peng Li | Organic compounds |
| US20120071450 * | Dec 7, 2009 | Mar 22, 2012 | Peng Li | Organic compounds |
| US20120238589 * | Sep 20, 2012 | Peng Li | Organic compounds |
| WO2014205354A3 * | Jun 20, 2014 | May 28, 2015 | Takeda Pharmaceutical Company Limited | Free base crystals |
| WO2015196186A1 * | Jun 22, 2015 | Dec 23, 2015 | Intra-Cellular Therapies, Inc. | Organic compounds |
| US8829008 | Jun 1, 2012 | Sep 9, 2014 | Takeda Pharmaceutical Company Limited | Organic compounds |
| US9000001 | Jul 18, 2012 | Apr 7, 2015 | Intra-Cellular Therapies, Inc. | Organic compounds |
| US9006258 | Dec 5, 2007 | Apr 14, 2015 | Intra-Cellular Therapies, Inc. | Method of treating female sexual dysfunction with a PDE1 inhibitor |
| US9073936 | Mar 13, 2014 | Jul 7, 2015 | Intra-Cellular Therapies, Inc. | Organic compounds |
| WO2009075784A1 * | Dec 6, 2008 | Jun 18, 2009 | Intra Cellular Therapies Inc | Organic compounds |
| WO2010065151A1 * | Dec 7, 2009 | Jun 10, 2010 | Intra-Cellular Therapies, Inc. | Organic compounds |
| WO2013192556A2 * | Jun 21, 2013 | Dec 27, 2013 | Intra-Cellular Therapies, Inc. | Salt crystal |
//////
O=C(C1=C(NC2=CC=CC=C2)N(CC3=CC=C(C4=NC(F)=CC=C4)C=C3)N=C1N56)N(C)C5=N[C@@]7([H])[C@]6([H])CCC7.O=P(O)(O)O
OR
Fc1cccc(n1)c2ccc(cc2)Cn7nc5N3C(=N[C@@H]4CCC[C@H]34)N(C)C(=O)c5c7Nc6ccccc6