
Gly-Pro-Glu
Synonym: GPE, Glycyl-prolyl-glutamic acid, (1-3)IGF-1
Pfizer (Originator)
Neuren Pharmaceuticals (Originator)
Neuren Pharmaceuticals (Originator)
Glypromate; glycine-proline-glutamate (neuroprotectant), Neuren
- CAS Number 32302-76-4
- Empirical Formula C12H19N3O6
- Molecular Weight 301.30
- Psychiatric Disorders (Not Specified)
Neurologic Drugs (Miscellaneous)
Cognition Disorders, Treatment of
Antiepileptic Drugs
Antidepressants Biochem/physiol Actions
GPE is a naturally occurring peptide fragment which had been in phase III clinical trials at Neuren Pharmaceuticals for use as prophylactic neuroprotection for patients undergoing coronary artery bypass graft (CABG) and valvuloplasty surgery. Although clinical evaluation in Australia continues, phase III trials evaluating the compound in the U.S. were discontinued based on negative results. The compound is found in normal brain tissue and, when injected intravenously, has been shown to act by multiple pathways to protect brain tissue from injury. The drug was originally developed by Pfizer, but rights were transferred to Neuren pursuant to a proprietary agreement between the companies.
When
amino acids join together (forming short groups called polypeptides, or
much longer chains called proteins) the amine group of one amino acid
joins with the carboxyl group of the next, making a peptide bond.
These bonds don’t ionise at different pHs, but can be hydrolised —
broken — reforming the amino acids. GPE is formed from the amino acids
glycine, proline and glutamic acid:


This tripeptide has 3 pH-sensitive groups, each with its own pKa.
What the university chemists needed to do was work out what form GPE is
in when it is active in the brain, what parts of the molecule are
critical to its effectiveness, and how to ‘tweak’ the molecule (by
changing the side chains) so that it will remain in the brain for longer
than the naturally-occurring substance. They also needed to make sure
the final compound passes through the blood-brain barrier (that
prevents most substances in the blood from entering and affecting the
brain). If possible, they also wanted a compound that could be taken in
pill form without being broken down in the stomach. It was also
essential that the compound was safe for people to take!
Neuren Pharmaceuticals
Neuren Pharmaceuticals
After initial work on GPE at the university, the research was passed to a spin-off research group called Neuren Pharmaceuticals Ltd, which takes compounds discovered by the University of Auckland and develops them into medicines. Neuren developed GPE intoGlypromate®
and are working with researchers in the US (including the US Military,
who have a keen interest in a medicine that will reduce brain damage
after head injuries) to test the compound on patients. There is
considerable interest in Glypromate® world-wide, because at present
there is nothing that reduces cell death after brain injuries. The
chances of winning a race are pretty high when you’re the only
competitor!Glypromate® is being tested on heart-bypass patients because
up to 70% of bypass patients are affected mentally after their surgery.
It’s thought that tiny clots form after the heart is restarted, and that
these travel to the brain and cause mini-strokes. Unlike
naturally-occurring strokes, or the brain damage caused by accident or
war, the bypass surgery is planned, so before and after tests can be
done on the patients to see exactly what effect the treatment has. Early
results look very promising.
Glypromate is just one of the compounds Neuren is working on. Others may develop into treatments for Multiple Sclerosis, Parkinson’s Disease or Alzheimer’s Disease as well as various kinds of cancer. The company’s links with overseas research groups mean that compounds developed in New Zealand are able to be tested in the US and gain the FDA approval which will allow them to be used in most countries in the world.
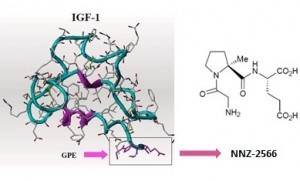
The tripeptide
Glycyl-L-Prolyl-L-Glutamate (Gly-Pro-Glu or GPE) is a naturally
occurring peptide, which is proteolytically cleaved from insulin-like
growth factor-1 (IGF-1). IGF-1 is a potent neurotrophic factor produced
endogenously in damaged regions of the brain. It has been postulated
that some of the neuroprotective actions of IGF-1 are mediated by GPE
although the precise mechanism of action remains unclear. GPE has a
different mode of action to IGF-1 as GPE does not bind to the IGF-1
receptor. Rather, GPE has been shown to bind with low affinity to the
N-methyl-D-aspartate (NMDA) receptor and also elicit a biological
response via other mechanisms. GPE facilitates the release of dopamine
through interaction with the NMDA receptor but GPE stimulated
acetylcholine release is via an unknown, non-NMDA pathway.Glypromate is just one of the compounds Neuren is working on. Others may develop into treatments for Multiple Sclerosis, Parkinson’s Disease or Alzheimer’s Disease as well as various kinds of cancer. The company’s links with overseas research groups mean that compounds developed in New Zealand are able to be tested in the US and gain the FDA approval which will allow them to be used in most countries in the world.
It has been demonstrated that GPE can act as a neuronal rescue agent following brain injury or disease, including hypoxic-ischemic brain injury, NMDA challenge, chemical toxins and in animal models of Parkinson's and Alzheimer's disease. Analogs of GPE are thus of interest in the development of novel pharmaceutical agents for the treatment of central nervous system (CNS) injuries and neurodegenerative disorders among others.
CURRENT STATUS
Neuren Pharmaceuticals
was developing Glypromate (glycine-proline glutamate), a naturally
occurring small-molecule neuroprotectant derived from IGF-1 which
inhibits caspase III dependent apoptosis, for the potential treatment of
neurodegenerative diseases by iv infusion. By June 2008, a phase III
trial had begun . However, in December 2008, the company discontinued
further development of the drug after it failed to show an observable
effect [972907]. In November 2005, the company was seeking to outlicense the drug [771417].
Neuren is also investigating the Glypromate analog, NNZ-2566 for similar indications.
Neuren is also investigating the Glypromate analog, NNZ-2566 for similar indications.
The proposed study will be an investigator-initiated study which means that the Investigational New Drug (IND) application will be submitted to the FDA by the Army investigators rather than by Neuren. Neuren will provide the drug product as well as access to preclinical, clinical and regulatory documents related to Glypromate(R). The Company's only financial commitment will be compensation to the Jackson Foundation for administrative costs incurred in coordinating the study. Neuren will retain all commercial rights to Glypromate(R) in these indications.
Cardiac arrest involves the sudden, complete cessation of heart function and circulation leading rapidly to neurological and other organ system damage. Among patients who survive, the consequences of neurological damage resulting from lack of blood flow and oxygen to the brain represent the primary adverse outcomes. This occurs in up to 80% of survivors and causes cognitive impairment such as occurs in patients undergoing major cardiac surgery, the focus for Neuren's upcoming Phase 3 study with Glypromate(R). However recovery without residual neurological damage after cardiac arrest is rare.
There are no drugs approved to reduce the neurological damage caused by cardiac arrest. Neuren believes that Glypromate(R) for this indication will be eligible for Orphan Drug designation. Orphan Drug designation provides for a period of market exclusivity following approval as well as possible access to US government grants. In addition, because of the serious nature of neurological impairment resulting from cardiac arrest and the lack of available drug therapy, Neuren intends to apply for Fast Track designation which provides for accelerated clinical development and review.
While the Army's investigator-initiated trial will focus on out of hospital cardiac arrest, if this trial is successful, Neuren, the Jackson Foundation and the Army investigators are considering additional trials of Glypromate(R) to reduce brain damage resulting from related conditions including in-hospital cardiac arrest and treatment of patients with ventricular fibrillation, the heart rhythm disturbance associated with more than 75% of cardiac arrests.
Under the agreement, the Jackson Foundation will provide support to the Army investigators in clinical trial preparations, protocol development, obtaining human subjects clearance, coordination of patient enrolment, data management and analysis, and preparation of study reports.
Mr David Clarke, CEO of Neuren said: "This is a very important development for Neuren in that it reflects a growing appreciation of the potential for Glypromate(R) to reduce neurological damage. It also, of course, reinforces the value and strength of Neuren's relationship with the US Army physicians and scientists. Cardiac arrest is a devastating clinical event and one for which a drug to reduce the neurological consequences is clearly needed. The addition of this trial will now give Neuren a very strong and cost effective portfolio of clinical trials in 2007 -- a Phase 3 and a Phase 2 for Glypromate(R) and the two Phase 2 trials with NNZ-2566."
Approximately 300,000 deaths result from cardiac arrest in the US each year, making cardiac arrest one of the leading causes of death. According to the American Heart Association, each year approximately 160,000 people in the US experience sudden cardiac arrest outside of a hospital or in a hospital emergency department.
Neuren estimates that the number of patients in the US that could be treated for out of hospital cardiac arrest and related indications is approximately 400,000 which could represent a potential market of US$800 million.
About Madigan Army Medical Center
Madigan Army Medical Center, located in Tacoma, Washington, is one of the major US Army medical centers, providing clinical care to over 120,000 active, reserve and retired military personnel and dependents. The hospital has a medical staff of more than 1,000 with 200 physicians and nurses in training. Madigan's Department of Clinical Investigations, which is dedicated to writing, performing, and regulating clinical research, is conducting approximately 200 clinical trials across a wide spectrum of indications from Phase I to IV.
About the Jackson Foundation
The Jackson Foundation is a private, not-for-profit organisation that supports the US military in conducting medical research and clinical trials and has established relationships with more than 160 military medical organisations worldwide. It was founded in 1983, in part, to foster cooperative relationships between the military medical community and the private sector, including pharmaceutical sponsors. The Jackson Foundation manages Phase I - IV clinical trials utilizing an established network of military medical centers across the United States.
About Glypromate(R)
Glypromate(R) is a peptide fragment of IGF-1 and is being developed by Neuren as a potential therapeutic candidate for diseases caused by some forms of chronic or acute brain injury. Glypromate(R) has been shown to act by multiple pathways to protect brain tissue from injury. Neuren has successfully completed a Phase I safety study and a Phase IIa safety and pharmacokinetics study and plans to initiate a Phase III study in late 2006.
About Neuren Pharmaceuticals
Neuren Pharmaceuticals is a biotechnology company developing novel therapeutics in the fields of brain injury and diseases and metabolic disorders. The Neuren portfolio consists of six product families, targeting markets with large unmet needs and limited competition. Neuren has three lead candidates, Glypromate(R) andNNZ-2566, presently in the clinic in development to treat a range of acute neurological conditions, and NNZ-2591, in preclinical development for Parkinson's and other chronic conditions. Neuren has commercial and development partnerships with the US ArmyWalter Reed Army Institute of Research, Metabolic Pharmaceuticals,UCLA Medical Center and the National Trauma Research Institute in Melbourne.
For more information, please visit Neuren's website at http://www.neurenpharma.com
Company David Clarke CEO of Neuren T: 1800 259 181 (Australia) T: +64 9 3 367 7167 ext 82308 (New Zealand) M: +64 21 988 052 Media and investor relations Rebecca Piercy Buchan Consulting T: +61 9827 2800 M: +61 422 916 422
CONTACT: David Clarke, CEO of Neuren, 1-800-259-181(Australia), or
+64-9-3-367-7167 ext 82308 (New Zealand), or +64-21-988-052 (mobile); or
Media and investor relations - Rebecca Piercy of Buchan Consulting,
+61-9827-2800, +61-422-916-422 (mobile)
Web site: http://www.neurenpharma.com/
REFERENCES
1 EP 0366638
2 WO 2005042000
3 WO 2008153929
4 WO 2009033805
5 WO 2009033806
Synthesis off isotopically labelled glycyl-L-prolyl-L-glutamic acid (Glypromate(R)) and derivatives
J Label Compd Radiopharm 2006, 49(6): 571
An efficient fmoc solid-phase synthesis of an amphiphile of the neuroprotective agent glycyl-prolyl-glutamic acid
Synlett (Stuttgart) 2014, 25(15): 2221
Intracellular pathways activated by Insulin-like growth factor 1 and its derivates
40th Annu Meet Soc Neurosci (November 13-17, San Diego) 2010, Abst 167.13
| EP2667715A1 * | Jan 27, 2012 | Dec 4, 2013 | Neuren Pharmaceuticals Limited | Treatment of autism spectrum disorderes using glycyl-l-2-methylprolyl-l-glutamic acid |
| EP2667715A4 * | Jan 27, 2012 | Jul 23, 2014 | Neuren Pharmaceuticals Ltd | Treatment of autism spectrum disorderes using glycyl-l-2-methylprolyl-l-glutamic acid |
| US8940732 | Jan 15, 2010 | Jan 27, 2015 | Massachusetts Institute Of Technology | Diagnosis of autism spectrum disorders and its treatment with an antagonist or inhibitor of the 5-HT2c receptor signaling pathway |
| US9212204 | Jan 26, 2015 | Dec 15, 2015 | Neuren Pharmaceuticals Limited |
| WO2005042000A1 * | 22 Oct 2004 | 12 May 2005 | David Charles Batchelor | Neuroprotective effects of gly-pro-glu following intravenous infusion |
| WO2005097161A2 * | 30 Mar 2005 | 20 Oct 2005 | Peter D Gluckman | Gpe and g-2mepe, caffeine and alkanol for treatment of cns injury |
| WO2006127702A2 * | 23 May 2006 | 30 Nov 2006 | Neuren Pharmaceuticals Ltd | Analogs of glycyl-prolyl-glutamate |
| EP0366638A2 * | 24 Oct 1989 | 2 May 1990 | KabiGen AB | Neuromodulatory peptide |
| US20020151522 * | 13 Mar 2002 | 17 Oct 2002 | Tajrena Alexi | Regulation of weight |
| Reference | ||
|---|---|---|
| 1 | * | ALONSO DE DIEGO, SERGIO A. ET AL: "New Gly-Pro-Glu (GPE) analogues: Expedite solid-phase synthesis and biological activity" BIOORGANIC & MEDICINAL CHEMISTRY LETTERS, vol. 16, no. 5, 2006, - 1392 page 1396, XP002527092 |
| 2 | * | SARA V R ET AL: "IDENTIFICATION OF GLY-PRO-GLU (GPE), THE AMINOTERMINAL TRIPEPTIDE OF INSULIN-LIKE GROWTH FACTOR 1 WHICH IS TRUNCATED IN BRAIN, AS A NOVEL NEUROACTIVE PEPTIDE" BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS, ACADEMIC PRESS INC. ORLANDO, FL, US, vol. 165, no. 2, 15 December 1989 (1989-12-15), pages 766-771, XP000992688 ISSN: 0006-291X |
//////Gly-Pro-Glu, GPE, Glycyl-prolyl-glutamic acid, 32302-76-4, Tripeptide, Glycyl-L-Prolyl-L-Glutamate, Glypromate®, (1-3)IGF-1 , PHASE 3, Glypromate, glycine-proline-glutamate, neuroprotectant, Neuren
Neuren’s NNZ-2566 shows clinical benefit in Rett syndrome trial


Promising results in Phase 2 clinical trial
by Michael Tranfaglia, MDFRAXA Medical Director
 This isn’t a Fragile X trial, but the Neuren compound, NNZ-2566, that is in trials now for Fragile X has shown significant positive effects in a Phase 2 trial for Rett syndrome.
This isn’t a Fragile X trial, but the Neuren compound, NNZ-2566, that is in trials now for Fragile X has shown significant positive effects in a Phase 2 trial for Rett syndrome.The results of the trial are interesting, in that improvement was seen a Rett syndrome-specific rating scale compared to placebo, and there was also improvement noted on the CGI-I (Clinical Global Impression of Improvement) and Caregiver Top 3 Concerns. However, there was no effect seen on ABC scores (Aberrant Behavior Checklist) compared to placebo. Many in the Fragile X field have noted the inadequacies of the ABC; indeed, it was never designed or intended to be an outcome measure for clinical trials. In this case, a Rett-specific rating scale called the Motor-Behavior Assessment (MBA) showed a statistically significant and clinically meaningful treatment effect at the highest dose of the Neuren compound compared to placebo.
This is great news for those of us in the Fragile X community for several reasons:
- It shows that this compound really does something—it seems to have useful properties in actual patients, and that’s not trivial.
- It demonstrates that disease-specific symptoms can improve significantly on the drug, and that improvement can be measured in a relatively short clinical trial.
- It shows that a drug can have beneficial effects on core features of a genetically based developmental disorder, even if the more general rating scales (like the ABC) show no change.
This last point is strongly reminiscent of the experience of many families and clinicians in recent Fragile X clinical trials, where the drugs showed no advantage compared to placebo based on rating scales, but genuine improvement was noted in many subjects, with significant deterioration upon discontinuation of the drugs. Thus the calls for improved rating scales which can “capture” these core, disease-specific therapeutic effects. The NeurenFragile X trial is using some Fragile X-specific outcome measures which will hopefully lead to similar positive results.
The fact that this result is good news for Neuren also means that the company should remain financially viable for longer, so that they can continue the development of this compound for a number of indications—more “shots on goal”.
Of course, the usual caveats apply: this was a small study, and these results need to be replicated in a larger Phase 3 trial. Still, there’s a realistic possibility that we may see a similar result in Fragile X!