
PF 04991532
GKA PF-04991532
(
S)-6-{3-cyclopentyl-2-[4-(trifluoromethyl)-1
H-imidazol-1-yl]propanamido}nicotinic acid
(S)-6-(3-Cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanamido)nicotinic Acid
(S)-6-(3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanamido)nicotinic acid
MW 396.36, MF C
18 H
19 F
3 N
4 O
3
CAS 1215197-37-7
3-Pyridinecarboxylic acid, 6-[[(2
S)-3-cyclopentyl-1-oxo-2-[4-(trifluoromethyl)-1
H-imidazol-1-yl]propyl]amino]-
http://www.biochemj.org/content/441/3/881
Type 2 diabetes mellitus (T2DM) is a rapidly expanding public
epidemic affecting over 300 million people worldwide. This disease is
characterized by elevated fasting plasma glucose (FPG), insulin
resistance, abnormally elevated hepatic glucose production (HGP), and
reduced glucose-stimulated insulin secretion (GSIS). Moreover, long-term
lack of glycemic control increases risk of complications from
neuropathic, microvascular, and macrovascular diseases.
The standard of care for T2DM is metformin followed by sulfonylureas,
dipeptidyl peptidase-4 (DPP-IV) inhibitors, and thiazolidinediones
(TZD) as second line oral therapies. As disease progression continues,
patients typically require injectable agents such as glucagon-like
peptide-1 (GLP-1) analogues and, ultimately, insulin to help maintain
glycemic control. Despite these current therapies, many patients still
remain unable to safely achieve and maintain tight glycemic control,
placing them at risk of diabetic complications and highlighting the need
for novel therapeutic options.

Glucokinase (hexokinase IV) continues to be a compelling target for
the treatment of type 2 diabetes given the wealth of supporting human
genetics data and numerous reports of robust clinical glucose lowering
in patients treated with small molecule allosteric activators. Recent
work has demonstrated the ability of hepatoselective activators to
deliver glucose lowering efficacy with minimal risk of hypoglycemia.
While orally administered agents require a considerable degree of
passive permeability to promote suitable exposures, there is no such
restriction on intravenously delivered drugs. Therefore, minimization of
membrane diffusion in the context of an intravenously agent should
ensure optimal hepatic targeting and therapeutic index.
Diabetes is a major public health concern because of its increasing
prevalence and associated health risks. The disease is characterized by
metabolic defects in the production and utilization of carbohydrates
which result in the failure to maintain appropriate blood glucose
levels. Two major forms of diabetes are recognized. Type I diabetes, or
insulin-dependent diabetes mellitus (IDDM), is the result of an absolute
deficiency of insulin. Type II diabetes, or non-insulin dependent
diabetes mellitus (NIDDM), often occurs with normal, or even elevated
levels of insulin and appears to be the result of the inability of
tissues and cells to respond appropriately to insulin. Aggressive
control of NIDDM with medication is essential; otherwise it can progress
into IDDM.
As blood glucose increases, it is transported into pancreatic beta
cells via a glucose transporter. Intracellular mammalian glucokinase
(GK) senses the rise in glucose and activates cellular glycolysis, i.e.
the conversion of glucose to glucose-6-phosphate, and subsequent insulin
release. Glucokinase is found principally in pancreatic β-cells and
liver parenchymal cells. Because transfer of glucose from the blood into
muscle and fatty tissue is insulin dependent, diabetics lack the
ability to utilize glucose adequately which leads to undesired
accumulation of blood glucose (hyperglycemia). Chronic hyperglycemia
leads to decreases in insulin secretion and contributes to increased
insulin resistance. Glucokinase also acts as a sensor in hepatic
parenchymal cells which induces glycogen synthesis, thus preventing the
release of glucose into the blood. The GK processes are thus critical
for the maintenance of whole body glucose homeostasis.
It is expected that an agent that activates cellular GK will
facilitate glucose-dependent secretion from pancreatic beta cells,
correct postprandial hyperglycemia, increase hepatic glucose utilization
and potentially inhibit hepatic glucose release. Consequently, a GK
activator may provide therapeutic treatment for NIDDM and associated
complications, inter alia, hyperglycemia, dyslipidemia, insulin
resistance syndrome, hyperinsulinemia, hypertension, and obesity.
Several drugs in five major categories, each acting by different
mechanisms, are available for treating hyperglycemia and subsequently,
NIDDM (Moller, D. E., “New drug targets for Type II diabetes and the
metabolic syndrome”
Nature 414; 821-827, (2001)): (A) Insulin
secretogogues, including sulphonyl-ureas (e.g., glipizide, glimepiride,
glyburide) and meglitinides (e.g., nateglidine and repaglinide) enhance
secretion of insulin by acting on the pancreatic beta-cells. While this
therapy can decrease blood glucose level, it has limited efficacy and
tolerability, causes weight gain and often induces hypoglycemia. (B)
Biguanides (e.g., metformin) are thought to act primarily by decreasing
hepatic glucose production. Biguanides often cause gastrointestinal
disturbances and lactic acidosis, further limiting their use. (C)
Inhibitors of alpha-glucosidase (e.g., acarbose) decrease intestinal
glucose absorption. These agents often cause gastrointestinal
disturbances. (D) Thiazolidinediones (e.g., pioglitazone, rosiglitazone)
act on a specific receptor (peroxisome proliferator-activated
receptor-gamma) in the liver, muscle and fat tissues. They regulate
lipid metabolism subsequently enhancing the response of these tissues to
the actions of insulin. Frequent use of these drugs may lead to weight
gain and may induce edema and anemia. (E) Insulin is used in more severe
cases, either alone or in combination with the above agents.
Ideally, an effective new treatment for NIDDM would meet the
following criteria: (a) it would not have significant side effects
including induction of hypoglycemia; (b) it would not cause weight gain;
(c) it would at least partially replace insulin by acting via
mechanism(s) that are independent from the actions of insulin; (d) it
would desirably be metabolically stable to allow less frequent usage;
and (e) it would be usable in combination with tolerable amounts of any
of the categories of drugs listed herein.
Substituted heteroaryls, particularly pyridones, have been implicated
in mediating GK and may play a significant role in the treatment of
NIDDM. For example, U.S. Patent publication No. 2006/0058353 and PCT
publication Nos. WO2007/043638, WO2007/043638, and WO2007/117995 recite
certain heterocyclic derivatives with utility for the treatment of
diabetes. Although investigations are on-going, there still exists a
need for a more effective and safe therapeutic treatment for diabetes,
particularly NIDDM.

PATENT
US 20100063063
http://www.google.com/patents/US20100063063
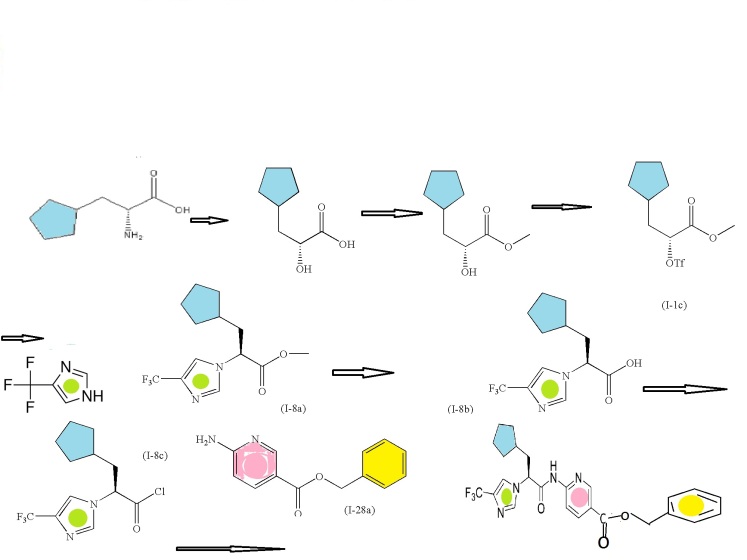
SYNTHESIS CONSTRUCTION

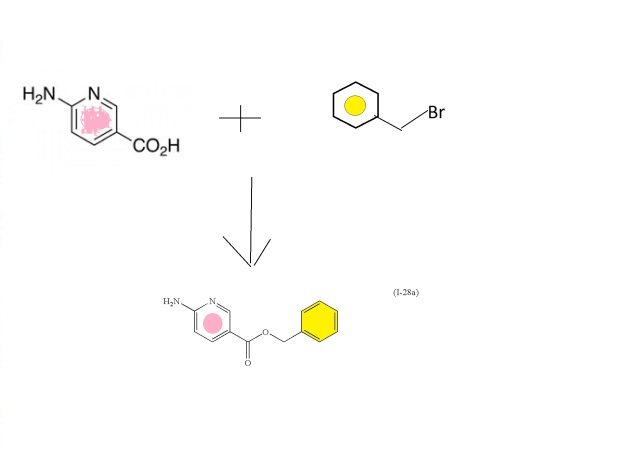
6-aminonicotinic acid

BENZYL BROMIDE

FIRST KEY INTERMEDIATE
SECOND SERIES FOR NEXT INTERMEDIATE

(R)-2-amino-3-cyclopentylpropanoic acid

(R)-methyl 3-cyclopentyl-2-hydroxypropanoic acid (I-1a)

(R)-methyl 3-cyclopentyl-2-hydroxypropanoate (I-1b)

Trifluoromethanesulfonic acid anhydride

(R)-methyl 3-cyclopentyl-2-(trifluoromethylsulfonyloxy)propanoate (I-1c)
CONDENSED WITH

4-Trifluoromethyl-1H-imidazole
TO GIVE PRODUCT SHOWN BELOW

(S)-methyl 3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanoate (I-8a)

(S)-3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanoic acid (I-8b)

CONVERTED TO ACID CHLORIDE, (S)-3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanoyl chloride (I-8c)
AND CONDENSED WITH

WILL GIVE BENZYL DERIVATIVE AS BELOW

THEN DEBENZYLATION TO FINAL PRODUCT

Intermediate: (R)-methyl 3-cyclopentyl-2-hydroxypropanoic acid (I-1a)

To a stirred solution of (R)-2-amino-3-cyclopentylpropanoic acid (5.0
grams; Chem-Impex International, Inc., Wood Dale, Ill.) and 1 M H
2SO
4 (45.1 mL) at 0° C., was added a solution of NaNO
2 (3.12 g) in H
2O
(15.6 mL) drop wise over 10 minutes. The reaction mixture was stirred
for 3 hours at 0° C., then for 2 hours at room temperature. The solution
was then extracted (3 times) with diethyl ether. The combined organic
extracts were dried over MgSO
4, filtered, and the filtrate concentrated to afford 2.36 g of (I-1a).
1H NMR (400 MHz, CDCl
3) δ 4.26-4.28 (1H), 1.99-2.07 (1H), 1.76-1.81 (4H), 1.60-1.62 (4H), 1.12-1.16 (2H); LCMS for C
8H
14O
3 m/z 157.1 (M−H)
−.
Intermediate: (R)-methyl 3-cyclopentyl-2-hydroxypropanoate (I-1b)

To a stirred solution of 2.36 g of (I-1a) in anhydrous methanol (15 mL) at room temperature was added SOCl
2(1.64
mL). The resulting mixture was heated at reflux for 2 hours. It was
then cooled and concentrated under reduced pressure. The residue was
partitioned between ethyl acetate and aqueous saturated NaHCO
3 solution.
The biphasic mixture was separated and the aqueous portion was
extracted with ethyl acetate. The combined extracts were dried over MgSO
4,
filtered, and the filtrate concentrated under reduced pressure. The
resulting residue was purified by flash column chromatography (silica
gel, heptanes/ethyl acetate) to afford 1.5 g of (I-1b) as a clear oil.
1H NMR (400 MHz, CDCl
3) δ 4.15-4.20 (1H), 3.77 (3H), 2.62-2.63 (1H), 1.97-2.05 (1H), 1.49-1.86 (8H), 1.06-1.17 (2H); LCMS for C
9H
16O
3 m/z 171.6 (M)
+. Intermediate (I-1b) can alternatively be prepared by the method described below.
A 0.2M solution of Li
2CuCl
4 was prepared as follows: Anhydrous CUCl
2 (26.9
g, 200 mol) and anhydrous LiCl (17.0 g, 400 mmol) were dissolved in THF
(1000 mL). The mixture required gentle heating to completely dissolve
the solids. After cooling the solution is ready for use.
A solution of Li
2CuCl
4 (0.2 M in THF, 125 mL,
25.0 mmol) was added slowly to a suspension of cyclopentylmagnesium
bromide (2 M in diethyl ether, 135 mL, 270 mmol; Aldrich Chemical
Company, Inc., Milwaukee, Wis.) and THF (500 mL) at −50° C. over 2-3
mins. The pale grey/brown suspension was then allowed to warm slowly to
−10° C. over 30 mins, by which time the color had developed to a dark
grey. The mixture was re-cooled to −78° C. and (R)-methyl
oxirane-2-carboxylate (25.0 g, 245 mmol; Aldrich Chemical Company, Inc.,
Milwaukee, Wis.) was added neat via syringe over 90 seconds. The
reaction was then stirred at −78° C. for 20 mins, before removing the
ice-bath and allowing to warm to approximately −50° C. over 30 mins.
Saturated NH
4Cl (aq, 700 mL) was then added and the mixture
stirred for 30 mins. The organic layer was collected and the aqueous
layer extracted with diethyl ether (2×250 mL). The combined organics
were washed with saturated NH
4Cl (aq, 350 mL), dried over MgSO
4,
and evaporated. Distillation of the crude residue (68-70° C. at 0.8
mbar) yielded 65-70% of (I-1b) as a pale yellow oil. A small amount of
less volatile material remained in the still pot.
1H NMR (400 MHz; CDCl
3): δ 4.17(1H), 3.76 (3H), 2.67 (1H), 2.01 (1H), 1.48-1.88 (8H), 1.11 (2H).
Intermediate: (R)-methyl 3-cyclopentyl-2-(trifluoromethylsulfonyloxy)propanoate (I-1c)
Intermediate: (R)-methyl 3-cyclopentyl-2-(trifluoromethylsulfonyloxy)propanoate (I-1c

Intermediate (I-1b) (6.37 g, 37.0 mmol) was dissolved in dry
dichloromethane (260 mL) and stirred under nitrogen in an ice bath.
2,6-Lutidine (9.0 mL, 77 mmol) was added. Trifluoromethanesulfonic acid
anhydride (11 mL, 65 mmol) in dry dichloromethane (75 mL) was added
dropwise. The reaction was stirred in the ice bath for 60 minutes,
concentrated under reduced pressure, and taken up in 1N HCl and methyl
t-butyl ether. The aqueous layer was separated, and the organic layer
was washed with additional 1N HCl to insure the removal of all the
lutidine. The combined organic layer was then washed with brine, dried
over sodium sulfate, filtered, concentrated under reduced pressure, and
dried under high vacuum to afford (I-1c) (11.3 g, 37 mmol, 100%), which
was used immediately without further purification;
1H NMR (400 MHz, CDCl
3) δ 5.10-5.14 (1H), 3.82 (3H), 2.02-2.12 (1H), 1.79-1.98 (4H), 1.51-1.66 (4H), 1.08-1.18 (2H).
Intermediate (I-1b) (6.37 g, 37.0 mmol) was dissolved in dry
dichloromethane (260 mL) and stirred under nitrogen in an ice bath.
2,6-Lutidine (9.0 mL, 77 mmol) was added. Trifluoromethanesulfonic acid
anhydride (11 mL, 65 mmol) in dry dichloromethane (75 mL) was added
dropwise. The reaction was stirred in the ice bath for 60 minutes,
concentrated under reduced pressure, and taken up in 1N HCl and methyl
t-butyl ether. The aqueous layer was separated, and the organic layer
was washed with additional 1N HCl to insure the removal of all the
lutidine. The combined organic layer was then washed with brine, dried
over sodium sulfate, filtered, concentrated under reduced pressure, and
dried under high vacuum to afford (I-1c) (11.3 g, 37 mmol, 100%), which
was used immediately without further purification;
1H NMR (400 MHz, CDCl
3) δ 5.10-5.14 (1H), 3.82 (3H), 2.02-2.12 (1H), 1.79-1.98 (4H), 1.51-1.66 (4H), 1.08-1.18 (2H)
Intermediate: (S)-methyl 3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanoate (I-8a)

4-Trifluoromethyl-1H-imidazole (5.0 g, 37.0 mmol; Apollo Scientific
Ltd., Bredbury, Cheshire, UK) was stirred in dry THF (180 mL) under
nitrogen at room temperature. Lithium hexamethyldisilazide (1M in THF,
33.4 mL, 33.4 mmol) was added dropwise via addition funnel. The mixture
was stirred at room temperature for 50 minutes and then chilled in an
ice bath. A solution of (I-1c) (11.3 g, 37 mmol) in dry THF (45 mL),
which had been chilled in an ice bath, was added in one portion. The
reaction was allowed to warm to room temperature, stirred for 2 hours,
quenched with saturated aqueous ammonium chloride solution (20 mL) and
allowed to stir overnight. The aqueous layer was separated, and the
organic layer was concentrated and then diluted with water and ethyl
acetate. The organic layer was washed in series with dilute aqueous
phosphoric acid, aqueous 10% potassium carbonate, and brine. The organic
layer was then dried over sodium sulfate, filtered, and concentrated
under reduced pressure to a brown oil. The crude material, containing
the undesired regioisomer as a small impurity, was purified by
chromatography on a 330 g pre-packed silica gel column, eluting with 10%
ethyl acetate/heptane, linear gradient to 70% ethyl acetate/heptane.
The product fractions were located by spotting on a silica TLC plate and
visualizing with KMnO
4 stain. TLC (1:1 ethyl
acetate/heptane, developed in potassium permanganate) located the pure
and mixed fractions. The clean product fractions were combined,
evaporated, and dried under high vacuum to afford (I-8a) as a clear oil
(6.61 g, 22.4 mmol, 67%).
1H NMR (400 MHz, CDCl
3) δ 7.57 (1H), 7.38 (1H), 4.71-4.74 (1H), 3.76 (3H), 2.01-2.14 (2H), 1.45-1.79 (7H), 1.03-1.18 (2H); m/z 291.4 (M+H)
+.
Intermediate: (S)-3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanoic acid (I-8b

6N HCl (140 mL) was added to (I-8a) (6.61 g, 22.4 mmol) and the
mixture was warmed to 95° C. for 16 hours and then allowed to cool.
Solid potassium carbonate (58 g) was added in portions to bring the pH
to about 4. A precipitate crashed out. Ethyl acetate was added, and the
mixture was stirred until everything dissolved. The aqueous layer was
extracted once with ethyl acetate. The combined organics were washed
with brine, dried over sodium sulfate, filtered, concentrated under
reduced pressure, and dried under high vacuum to afford (I-8b) as a
clear glass (6.15 g, 21.9 mmol, 98%).
1H NMR (400 MHz, CDCl
3) δ 7.73 (1H), 7.34 (1H), 6.85-7.15 (1H), 4.66-4.70 (1H), 1.98-2.17 (2H), 1.41-1.75 (7H), 1.01-1.19 (2H); m/z 277.4 (M+H)
+.
Intermediate: (S)-3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanoyl chloride (I-8c)

To a suspension of intermediate (I-8b) (0.25 g, 0.9 mmol) in
dichloromethane (5 mL) was added oxalyl chloride (0.35 g, 2.7 mmol) and
N,N-dimethylformamide (1 drop) at room temperature. The mixture was
stirred for 2 hours at room temperature. The reaction mixture was
concentrated in vacuo, and the residue was chased with dichloromethane
two times and concentrated in vacuo to afford (I-8c) (0.27 g, 100%) as
an oil, which was used in the next step directly.
Intermediate: (S)-6-(3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanamido) nicotinoyl chloride (I-21a)
-
Thionyl chloride (225 mg, 1.89 mmol) was added
to a solution of the compound of Example 48 (150 mg, 0.387 mmol) in
dichloromethane (1.5 mL) and the reaction stirred at room temperature
for 1 hour. LCMS of an aliquot in methanol showed ˜67% methyl ester. To
the reaction mixture was added another 25 uL of thionyl chloride and
this was stirred at room temp for another 30 minutes. Solvents were
evaporated to afford 157 mg (100%) of (I-21a) as a grayish-white solid.
LCMS in methanol to generate the methyl ester gave m/z 395.9 (M+H)+.
(I-8b
Intermediate: (S)-3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanoic acid (I-8b)

6N HCl (140 mL) was added to (I-8a) (6.61 g, 22.4 mmol) and the
mixture was warmed to 95° C. for 16 hours and then allowed to cool.
Solid potassium carbonate (58 g) was added in portions to bring the pH
to about 4. A precipitate crashed out. Ethyl acetate was added, and the
mixture was stirred until everything dissolved. The aqueous layer was
extracted once with ethyl acetate. The combined organics were washed
with brine, dried over sodium sulfate, filtered, concentrated under
reduced pressure, and dried under high vacuum to afford (I-8b) as a
clear glass (6.15 g, 21.9 mmol, 98%).
1H NMR (400 MHz, CDCl
3) δ 7.73 (1H), 7.34 (1H), 6.85-7.15 (1H), 4.66-4.70 (1H), 1.98-2.17 (2H), 1.41-1.75 (7H), 1.01-1.19 (2H); m/z 277.4 (M+H)
+.
(I-28a
Intermediate: benzyl 6-aminonicotinate (I-28a)

To a stirred suspension of 6-aminonicotinic acid (100 g, 0.72 mol;
Aldrich Chemical Company, Inc., Milwaukee, Wis.) in
N,N-dimethylformamide (700 mL) with brisk mechanical stirring was added
potassium carbonate (150 g, 1.08 mol) and the reaction was stirred for
10 min before the portionwise addition of benzyl bromide (95 mL, 0.80
mol). The reaction was stirred at room temperature overnight, then the
solids were filtered off and washed thoroughly with ethyl acetate, and
the solvent was removed under vacuum. The filter cake was dissolved in
water and extracted with ethyl acetate. The residue after evaporation of
N,N-dimethylformamide was combined with the ethyl acetate extracts
(total volume 2 L of ethyl acetate) and the combined organic extracts
washed with brine (5×500 mL), dried (MgSO
4) and the solvent
removed under reduced pressure. The crude product was refluxed with 1:1
diethyl ether:hexane for 30 min then the solids filtered off (warm),
washed with diethyl ether:hexane (1:1), and dried. This solid was
precipitated from hot toluene (hot filtration required to remove
dibenzylated material) and dried to afford (I-28a) (107.2 g, 65%) as an
off-white solid;
1H NMR (DMSO-d
6): δ 8.50 (1H), 7.82 (1H), 7.34-7.29 (5H), 6.84 (2H), 6.43 (1H), 5.23 (2H); m/z 229.4 (M+H)
+.
Example 47
(S)-benzyl 6-(3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanamido)nicotinate
Formula (1A-4) wherein R
4 is

To Intermediate (I-8b) (16.28 g, 59.8 mmol) stirring in dry
dichloromethane (400 mL) at room temperature under nitrogen was added 2
drops of DMF. Oxalyl chloride (11 mL, 130 mmol) was added dropwise.
After the bubbling subsided the reaction was left stirring for 90
minutes and then concentrated under reduced pressure. Two successive
portions of 1,2-dichloroethane were added and evaporated to remove all
excess oxalyl chloride. The crude acid chloride was taken up in
dichloromethane (150 mL) and stirred at room temperature. Intermediate
(I-28a) (14.3 g, 62.5 mmol) and pyridine (10 mL, 130 mmol) were stirred
in 400 mL dry dichloromethane. This was added to the acid chloride
solution, using another 50 mL dry dichloromethane to complete the
transfer. The mixture was left stirring at room temperature under
nitrogen for 18 hours. The reaction was diluted with dichloromethane and
water, and 1M aqueous phosphoric acid was added. The organic layer was
separated and washed sequentially with dilute aqueous potassium
carbonate, and brine. This was then dried over sodium sulfate, filtered,
and concentrated under reduced pressure to a glass, which was taken up
in hot ethyl acetate and stirred at room temperature. A precipitate
appeared at about 30 minutes. The mixture was stirred for 16 hours and
then filtered. The precipitate was washed with ethyl acetate and then
diethyl ether and dried under high vacuum at 60° C. to afford the title
compound as a white solid (17.8 g, 36.6 mmol, 61%). The mother liquor
was evaporated and purified by silica gel chromatography on a 120 g
pre-packed column, eluting with 40% ethyl acetate/heptane. The product
fractions were combined, concentrated under reduced pressure, dried
under high vacuum to a glass, and converted as previously described to
additional product (3.5 g, 7.2 mmol, 12%, total yield 73%).
1H NMR (400 MHz, DMSO-d
6)
δ 11.50 (1H), 8.87-8.88 (1H), 8.29-8.32 (1H), 8.12-8.14 (1H), 7.93-7.94
(2H), 7.39-7.46 (2H), 7.30-7.37 (3H), 5.32 (2H), 5.21-5.25 (1H),
2.06-2.19 (2H), 1.26-1.63 (8H), 1.01-1.06 (1H); m/z 487.5 (M+H)
+.
Example 48
(S)-6-(3-cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanamido)nicotinic acid
Formula (1A-4) wherein R
4 is

The compound of Example 47 (4.07 g, 8.35 mmol) was added to a 500 mL
Parr bottle, followed by ethyl acetate (50 mL) and ethanol (100 mL). The
mixture was warmed until all of the solid dissolved, and then cooled to
room temperature. 10% Pd/C (450 mg) was added, and the mixture was
shaken under 50 psi hydrogen for 90 minutes. The reaction was filtered
through a microfiber filter. The filtrate was concentrated under reduced
pressure and dried under high vacuum at 50° C. to afford product as a
glassy solid (3.0 g, 7.75 mmol, 90.6%). The glassy solid was stirred
overnight in diethyl ether. The white solid precipitate was filtered,
washed with diethyl ether, suction dried, and dried under high vacuum at
50° C. to afford the title compound as a white solid.
1H NMR (400 MHz, DMSO-d6)
δ 13.10-13.25 (1H), 11.44 (1H), 8.83 (1H), 8.23-8.26 (1H), 8.09-8.12
(1H), 7.94-7.95 (2H), 5.22-5.26 (1H), 2.06-2.17 (2H), 1.29-1.64 (8H),
1.04-1.07 (1H);
m/z 397.3 (M+H)+.
THIS NMR IS FROM SUPPORTING INFO OF A JOURNAL

 PAPER
PAPER
Organic Process Research & Development (2012), 16(10), 1635-1645
http://pubs.acs.org/doi/abs/10.1021/op300194c
This work describes the process
development and manufacture of early-stage clinical supplies of a
hepatoselective glucokinase activator, a potential therapy for type 2
diabetes mellitus. Critical issues centered on challenges associated
with the synthesis of intermediates and API bearing a particularly
racemization-prone α-aryl carboxylate functionality. In particular, a
T3P-mediated amidation process was optimized for the coupling of a
racemization-prone acid substrate and a relatively non-nucleophilic
amine. Furthermore, an unusually hydrolytically-labile amide in the API
also complicated the synthesis and isolation of drug substance. The
evolution of the process over multiple campaigns is presented, resulting
in the preparation of over 110 kg of glucokinase activator.
(S)-6-(3-Cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanamido)nicotinic Acid (1)
Pressure Hydrogenation
1 (89% yield) as a white solid:
mp 187–189 °C;
1H NMR (400 MHz, d6-DMSO)
δ 13.23 (s, 1H), 11.49 (s, 1H), 8.86 (dd, J = 0.4, 2.4 Hz, 1H), 8.27
(dd, J = 2.4, 8.8 Hz, 1H), 8.13 (d, J = 8.8 Hz, 1H), 7.97–7.99 (m, 2H),
5.27 (dd, J = 5.6, 10.0 Hz, 1H), 2.20 (ddd, J = 6.0, 10.0, 14.0, 1H),
2.10 (ddd, J = 5.6, 8.4, 14.0, 1H), 1.27–1.69 (m, 8H), 1.03–1.12 (m,
1H);
13C NMR (100 MHz, d6-DMSO) δ 168.8, 165.7, 154.3, 149.7, 139.6, 138.8, 129.9 (q, JCF = 38 Hz), 122.6, 122.0 (q, JCF = 265 Hz), 120.0 (q, JCF = 4 Hz), 112.8, 60.0, 37.6, 36.2, 32.0, 30.8, 24.6, 24.4;
19F NMR (376 MHz, d6-DMSO) δ −60.7.
HRMS-ESI m/z: [M + H]+ calcd for C18H19F3N4O3, 397.1482; found, 397.1481.
Achiral HPLC: rt 4.6 min. Chiral SFC: rt 4.1 min (1), 3.1 min (ent-1).
PAPER
Journal of Medicinal Chemistry (2012), 55(3), 1318-1333
http://pubs.acs.org/doi/abs/10.1021/jm2014887

Glucokinase is a key regulator of
glucose homeostasis, and small molecule allosteric activators of this
enzyme represent a promising opportunity for the treatment of type 2
diabetes. Systemically acting glucokinase activators (liver and
pancreas) have been reported to be efficacious but in many cases present
hypoglycaemia risk due to activation of the enzyme at low glucose
levels in the pancreas, leading to inappropriately excessive insulin
secretion. It was therefore postulated that a liver selective activator
may offer effective glycemic control with reduced hypoglycemia risk.
Herein, we report structure–activity studies on a carboxylic acid
containing series of glucokinase activators with preferential activity
in hepatocytes versus pancreatic β-cells. These activators were designed
to have low passive permeability thereby minimizing distribution into
extrahepatic tissues; concurrently, they were also optimized as
substrates for active liver uptake via members of the organic anion
transporting polypeptide (OATP) family. These studies lead to the
identification of 19 as a potent glucokinase activator with a
greater than 50-fold liver-to-pancreas ratio of tissue distribution in
rodent and non-rodent species. In preclinical diabetic animals, 19
was found to robustly lower fasting and postprandial glucose with no
hypoglycemia, leading to its selection as a clinical development
candidate for treating type 2 diabetes.
(S)-6-(3-Cyclopentyl-2-(4-(trifluoromethyl)-1H-imidazol-1-yl)propanamido)nicotinic Acid (19)
afford 19 as a white solid (3.22 g, 71%).
1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 8.86 (d, J = 1.95 Hz, 1H), 8.27 (dd, J = 2.24, 8.68 Hz, 1H), 8.13 (d, J = 8.78 Hz, 1H), 7.97 (d, J = 4.88 Hz, 2H), 5.27 (dd, J = 5.37, 9.66 Hz, 1H), 2.04–2.26 (m, 2H), 1.38–1.72 (m, 7H), 1.26–1.37 (m, 1H), 1.08 (td, J = 7.88, 11.75 Hz, 1H);
LCMS m/z 397.5 (M + H)+.
HPLC purity (method A): tR = 7.690 min, 100%.
PAPER
Bioorganic & Medicinal Chemistry Letters (2013), 23(24), 6588-6592
http://www.sciencedirect.com/science/article/pii/S0960894X13012638


Figure 1.
Structure of Hepatoselective GKA PF-04991532 (1).
References
Drug Metabolism & Disposition (2015), 43(2), 190-198
PLoS One (2014), 9(5), e97139/1-e97139/9,
Journal of Biological Chemistry (2012), 287(17), 13598-13610
Drug Discovery Today (2012), 17(9-10), 528-529
Biochemical Journal (2012), 441(3), 881-887.
///////////
Figure 1. Representative structures of glucokinase activators.









 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE amcrasto@gmail.com
amcrasto@gmail.com






