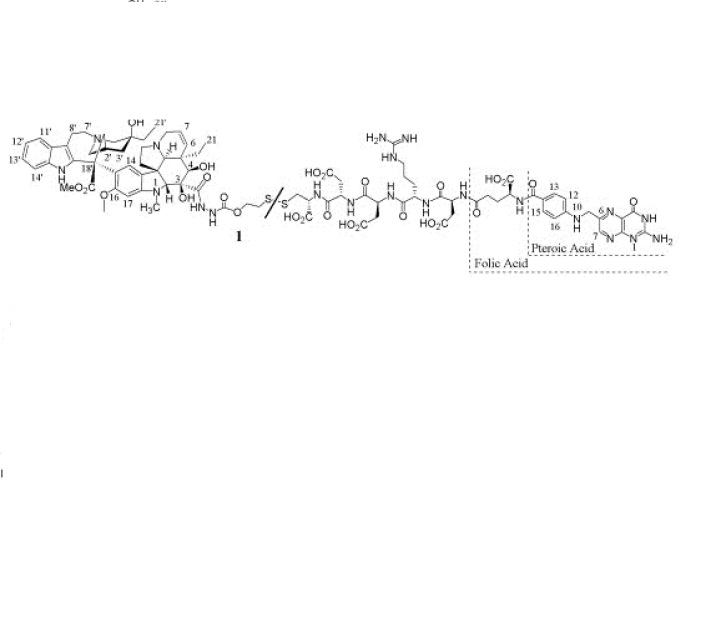
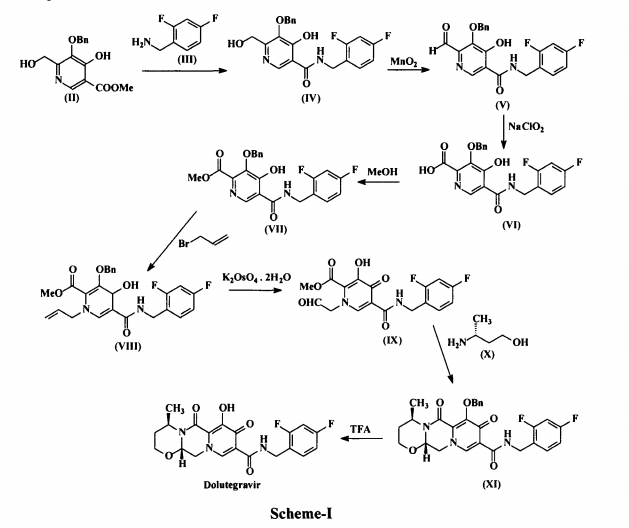
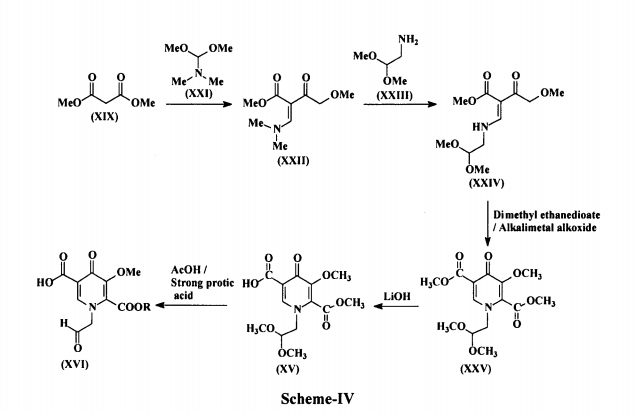
Duvelisib
INK 1197; IPI 145; 8-Chloro-2-phenyl-3-[(1S)-1-(9H-purin-6-ylamino)ethyl]-1(2H)-isoquinolinone
1(2H)-Isoquinolinone, 8-chloro-2-phenyl-3-((1S)-1-(9H-purin-6-ylamino)ethyl)-
8-Chloro-2-phenyl-3-((1S)-1-(7H-purin-6-ylamino)ethyl)isoquinolin-1(2H)-one
8-Chloro-2-phenyl-3-((1S)-1-(7H-purin-6-ylamino)ethyl)isoquinolin-1(2H)-one
(S)-3-(l-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-l(2H)-one
UNII-610V23S0JI; IPI-145; INK-1197;
Originator…….. Millennium Pharmaceuticals
| Molecular Formula | C22H17ClN6O | |
| Molecular Weight | 416.86 | |
| CAS Registry Number | 1201438-56-3 |

Infinity Pharmaceuticals has partnered with AbbVie to develop and commercialise its duvelisib (IPI-145), an oral inhibitor of phosphoinositide-3-kinase (PI3K)-delta and PI3K-gamma, to treat patients with cancer.

Duvelisib
The filing of patents claiming new crystalline forms, usually 4−6 years after the original product patent, is a typical strategy applied by such companies to extend patent protection. This patent protection approach by big pharma forces generic bulk producers to discover and file patents on new polymorphs if they want to market the drug after expiry of the product patents.
Polymorphism is of paramount importance due to its effect on some physical characteristics of powders such as melting point, flowability, vapour pressure, bulk density, chemical reactivity, apparent solubility and dissolution rate, and optical and electrical properties. In other words, polymorphism can affect drug stability, manipulation, and bioavailability
the principal aim of generic bulk producers was to generate a competitive market advantage by protecting their new crystal form.
An invention must:
A. be novel.
B. not be obvious for a person skilled in the art
C. be useful.
D. contain sufficient details to allow others to reproduce the invention.
Crystalline form patents represent a small but very important segment of product patents because of the possibility to extend the medicine market protection, thus delaying competition from generic firms. We think that for these specific types of patent applications, the following basic rules should be applied:
1. The crystalline form cannot be characterised by a single technique.
2. When a pharmaceutical application or advantage is claimed to justify the usefulness of the patent application, volatile impurities must comply with ICH guidelines,23 and the new crystalline form must be sufficiently stable to be used as a medicine.
3. A new polymorph must have an advantage over the one previously described. The claiming of a crystalline form or solvate without a clear understanding of the usefulness is common to several patent case studies. From our direct experience, an interesting example is Cabergoline (Parkinson's disease): the originator and generic companies claimed up to 14 crystalline forms and solvates.24 What is the meaning of all these patent applications? Where is the advantage with respect to the previously reported crystalline forms or solvates?
Polymorphic forms of a compound of Formula (I):.US8809349
herein referred to as Form A, Form B, Form C, Form D, Form E, Form F, Form G, Form H, Form I, Form J, or an amorphous form of a compound of Formula (I), or a salt, solvate, or hydrate thereof; or a mixture of two or more thereof. In one embodiment, the polymorphic form of a compound of Formula (I) can be a crystalline form, a partially crystalline form, an amorphous form, or a mixture of crystalline form(s) and/or amorphous form(s).
(XRPD) peaks
Polymorph Form A has the following characteristic X-ray Powder Diffraction (XRPD) peaks: 2θ=9.6° (±0.2°), 12.2° (±0.2°), and 18.3° (±0.2°);
polymorph Form B has the following characteristic XRPD peaks: 2θ=7.9° (±0.2°), 13.4° (±0.2°), and 23.4° (±0.2°);
polymorph Form C has the following characteristic XRPD peaks: 2θ=10.4° (±0.2°), 13.3° (±0.2°), and 24.3° (±0.2°);
polymorph Form D has the following characteristic XRPD peaks: 2θ=11.4° (±0.2°), 17.4° (±0.2°), and 22.9° (±0.2°);
polymorph Form E has the following characteristic XRPD peaks: 2θ=6.7° (±0.2°), 9.3° (±0.2°), and 24.4° (±0.2°);
polymorph Form F has the following characteristic XRPD peaks: 2θ=9.6° (±0.2°), 17.3° (±0.2°), and 24.6° (±0.2°);
polymorph Form G has the following characteristic XRPD peaks: 2θ=6.7° (±0.2°), 9.5° (±0.2°), and 19.0° (±0.2°);
polymorph Form H has the following characteristic XRPD peaks: 2θ=8.9° (±0.2°), 9.2° (±0.2°), and 14.1° (±0.2°);
polymorph Form I has the following characteristic XRPD peaks: 2θ=9.7° (±0.2°), 19.3° (±0.2°), and 24.5° (±0.2°); and
polymorph Form J has the following characteristic XRPD peaks: 2θ=9.1° (±0.2°), 17.3° (±0.2°), and 18.3° (±0.2°).
“Enantiomerically pure”
As used herein, and unless otherwise specified, the term “enantiomerically pure” means a stereomerically pure composition of a compound having one or more chiral center(s).
As used herein, and unless otherwise specified, the terms “enantiomeric excess” and “diastereomeric excess” are used interchangeably herein. In some embodiments, compounds with a single stereocenter can be referred to as being present in “enantiomeric excess,” and those with at least two stereocenters can be referred to as being present in “diastereomeric excess.” For example, the term “enantiomeric excess” is well known in the art and is defined as:
eea=(conc.ofa-conc.ofbconc.ofa+conc.ofb)×100
Thus, the term “enantiomeric excess” is related to the term “optical purity” in that both are measures of the same phenomenon. The value of ee will be a number from 0 to 100, zero being racemic and 100 being enantiomerically pure. A compound which in the past might have been called 98% optically pure is now more precisely characterized by 96% ee. A 90% ee reflects the presence of 95% of one enantiomer and 5% of the other(s) in the material in question.
Some compositions described herein contain an enantiomeric excess of at least about 50%, 75%, 90%, 95%, or 99% of the S enantiomer. In other words, the compositions contain an enantiomeric excess of the S enantiomer over the R enantiomer. In other embodiments, some compositions described herein contain an enantiomeric excess of at least about 50%, 75%, 90%, 95%, or 99% of the R enantiomer. In other words, the compositions contain an enantiomeric excess of the R enantiomer over the S enantiomer.
GRAPHS
FIG. 1 shows an X-ray powder diffraction (XRPD) for Polymorph Form A.
FIG. 2 shows an XRPD for Polymorph Form B.
FIG. 3 shows an XRPD for Polymorph Form C.
FIG. 4 shows an XRPD for Polymorph Form D.
FIG. 5 shows an XRPD for Polymorph Form E.
FIG. 6 shows an XRPD for Polymorph Form F.
FIG. 7 shows an XRPD for Polymorph Form G.
FIG. 8 shows an XRPD for Polymorph Form H.
FIG. 9 shows an XRPD for Polymorph Form I.
FIG. 10 shows an XRPD for Polymorph Form J.
FIG. 11 shows an XRPD for amorphous compound of Formula (I).
FIG. 12 shows a differential scanning calorimetry (DSC) thermogram for Polymorph Form A.
FIG. 13 shows a DSC for Polymorph Form B.
FIG. 14 shows a DSC for Polymorph Form C.
FIG. 15 shows a DSC for Polymorph Form D.
FIG. 16 shows a DSC for Polymorph Form E.
FIG. 17 shows a DSC for Polymorph Form F.
FIG. 18 shows a DSC for Polymorph Form G.
FIG. 19 shows a DSC for Polymorph Form H.
FIG. 20 shows a DSC for Polymorph Form I.
FIG. 21 shows a DSC for Polymorph Form J.
FIG. 22 shows a DSC thermogram and a thermogravimetric analysis (TGA) for Polymorph Form A.
FIG. 23 shows two DSC thermograms for Polymorph Form C.
FIG. 24 shows a DSC and a TGA for Polymorph Form F.
FIG. 25 shows a panel of salts tested for formation of crystalline solids in various solvents.
FIG. 26 shows a single crystal X-ray structure of Polymorph Form G MTBE (t-butyl methyl ether) solvate of a compound of Formula (I).
FIG. 27 shows an FT-IR spectra of Polymorph Form C.
FIG. 28 shows a 1H-NMR spectra of Polymorph Form C.
FIG. 29 shows a 13C-NMR spectra of Polymorph Form C.
FIG. 30 shows a dynamic vapor sorption (DVS) analysis of Polymorph Form C.
FIG. 31 shows representative dissolution profiles of capsules containing Polymorph Form C.
DRAWINGS
FIG. 1 shows an X-ray powder diffraction (XRPD) for Polymorph Form A.

FIG. 1 shows a representative X-ray powder diffraction (XRPD) for polymorph Form A.
In one embodiment, polymorph Form A can be characterized by any one, two, three, four, five, six, seven, eight, nine, ten, or more of significant peak(s) of FIG. 1. In one embodiment, polymorph Form A can be characterized as having at least one XRPD peak selected from 2θ=9.6° (±0.2°), 12.2° (±0.2°), and 18.3° (±0.2°). In one embodiment, polymorph Form A can be characterized as having at least one XRPD peak selected from 2θ=9.6° (±0.2°), 12.2° (±0.2°), and 18.3° (±0.2°) in combination with at least one XRPD peak selected from 2θ=15.6° (±0.2°) and 19.2° (±0.2°). In another embodiment, polymorph Form A can be characterized as having at least one XRPD peak selected from 2θ=9.6° (±0.2°), 12.2° (±0.2°), 15.6° (±0.2°), 18.3° (±0.2°), and 19.2° (±0.2°) in combination with at least one XRPD peak selected from 2θ=9.1° (±0.2°), 9.4° (±0.2°), 12.4° (±0.2°), 14.8° (±0.2°), 16.3° (±0.2°), 17.7° (±0.2°), 21.1° (±0.2°), 21.9° (±0.2°), 24.0° (±0.2°), and 26.9° (±0.2°). In one embodiment, polymorph Form A can be characterized in that it has substantially all of the peaks in its XRPD pattern as shown in FIG. 1.
In one embodiment, polymorph Form A can be characterized by any one, two, three, four, five, six, seven, eight, nine, ten, or more of significant peak(s) of FIG. 1. In one embodiment, polymorph Form A can be characterized as having at least one XRPD peak selected from 2θ=9.6° (±0.2°), 12.2° (±0.2°), and 18.3° (±0.2°). In one embodiment, polymorph Form A can be characterized as having at least one XRPD peak selected from 2θ=9.6° (±0.2°), 12.2° (±0.2°), and 18.3° (±0.2°) in combination with at least one XRPD peak selected from 2θ=15.6° (±0.2°) and 19.2° (±0.2°). In another embodiment, polymorph Form A can be characterized as having at least one XRPD peak selected from 2θ=9.6° (±0.2°), 12.2° (±0.2°), 15.6° (±0.2°), 18.3° (±0.2°), and 19.2° (±0.2°) in combination with at least one XRPD peak selected from 2θ=9.1° (±0.2°), 9.4° (±0.2°), 12.4° (±0.2°), 14.8° (±0.2°), 16.3° (±0.2°), 17.7° (±0.2°), 21.1° (±0.2°), 21.9° (±0.2°), 24.0° (±0.2°), and 26.9° (±0.2°). In one embodiment, polymorph Form A can be characterized in that it has substantially all of the peaks in its XRPD pattern as shown in FIG. 1.
FIG. 2 shows an XRPD for Polymorph Form B.

FIG. 2 shows a representative XRPD for polymorph Form B.
In one embodiment, polymorph Form B can be characterized by any one, two, three, four, five, six, seven, eight, nine, ten, or more of significant peak(s) of FIG. 2. In one embodiment, polymorph Form B can be characterized as having at least one XRPD peak selected from 2θ=7.9° (±0.2°), 13.4° (±0.2°), and 23.4° (±0.2°). In one embodiment, polymorph Form B can be characterized as having at least one XRPD peak selected from 2θ=7.9° (±0.2°), 13.4° (±0.2°), and 23.4° (±0.2°) in combination with at least one XRPD peak selected from 2θ=14.0° (±0.2°) and 15.0° (±0.2°). In another embodiment, polymorph Form B can be characterized as having at least one XRPD peak selected from 2θ=7.9° (±0.2°), 13.4° (±0.2°), 14.0° (±0.2°), 15.0° (±0.2°), and 23.4° (±0.2°) in combination with at least one XRPD peak selected from 2θ=9.5° (±0.2°), 12.7° (±0.2°), 13.6° (±0.2°), 14.2° (±0.2°), 15.7° (±0.2°), 19.0° (±0.2°), 22.3° (±0.2°), 24.2° (±0.2°), 24.8° (±0.2°), and 26.9° (±0.2°). In one embodiment, polymorph Form B can be characterized in that it has substantially all of the peaks in its XRPD pattern as shown in FIG. 2.
FIG. 3 shows an XRPD for Polymorph Form C.
In one embodiment, polymorph Form C can be characterized by any one, two, three, four, five, six, seven, eight, nine, ten, or more of significant peak(s) of FIG. 3. In one embodiment, Form C can be characterized by having at least one XRPD peak selected from 2θ=10.5° (±0.2°), 13.7° (±0.2°), and 24.5° (±0.2°). In another embodiment, Form C can be characterized by having at least one XRPD peak selected from 2θ=10.4° (±0.2°), 13.3° (±0.2°), and 24.3° (±0.2°). In one embodiment, polymorph Form C can be characterized as having at least one XRPD peak selected from 2θ=10.4° (±0.2°), 13.3° (±0.2°), and 24.3° (±0.2°) in combination with at least one XRPD peak selected from 2θ=6.6° (±0.2°) and 12.5° (±0.2°). In another embodiment, polymorph Form C can be characterized as having at least one XRPD peak selected from 2θ=6.6° (±0.2°), 10.4° (±0.2°), 12.5° (±0.2°), 13.3° (±0.2°), and 24.3° (±0.2°) in combination with at least one XRPD peak selected from 2θ=8.8° (±0.2°), 9.9° (±0.2°), 13.4° (±0.2°), 15.5° (±0.2°), 16.9° (±0.2°), 19.8° (±0.2°), 21.3° (±0.2°), 23.6° (±0.2°), 25.3° (±0.2°), and 27.9° (±0.2°). In one embodiment, polymorph Form C can be characterized in that it has substantially all of the peaks in its XRPD pattern as shown in FIG. 3.

FIG. 4 shows an XRPD for Polymorph Form D.

In one embodiment, polymorph Form D can be characterized by any one, two, three, four, five, six, seven, eight, nine, ten, or more of significant peak(s) of FIG. 4. In one embodiment, polymorph Form D can be characterized as having at least one XRPD peak selected from 2θ=11.4° (±0.2°), 17.4° (±0.2°), and 22.9° (±0.2°). In one embodiment, polymorph Form D can be characterized as having at least one XRPD peak selected from 2θ=11.4° (±0.2°), 17.4° (±0.2°), and 22.9° (±0.2°) in combination with at least one XRPD peak selected from 2θ=9.2° (±0.2°) and 18.3° (±0.2°). In another embodiment, polymorph Form D can be characterized as having at least one XRPD peak selected from 2θ=9.2° (±0.2°), 11.4° (±0.2°), 17.4° (±0.2°), 18.3° (±0.2°), and 22.9° (±0.2°) in combination with at least one XRPD peak selected from 2θ=9.8° (±0.2°), 12.2° (±0.2°), 15.8° (±0.2°), 16.2° (±0.2°), 16.8° (±0.2°), 18.9° (±0.2°), 19.9° (±0.2°), 20.0° (±0.2°), 24.9° (±0.2°), and 29.3° (±0.2°). In one embodiment, polymorph Form D can be characterized in that it has substantially all of the peaks in its XRPD pattern as shown in FIG. 4.
FIG. 5 shows an XRPD for Polymorph Form E. US8809349
In one embodiment, polymorph Form E can be characterized by any one, two, three, four, five, six, seven, eight, nine, ten, or more of significant peak(s) of FIG. 5. In one embodiment, polymorph Form E can be characterized as having at least one XRPD peak selected from 2θ=6.7° (±0.2°), 9.3° (±0.2°), and 24.4° (±0.2°). In one embodiment, polymorph Form E can be characterized as having at least one XRPD peak selected from 2θ=6.7° (±0.2°), 9.3° (±0.2°), and 24.4° (±0.2°) in combination with at least one XRPD peak selected from 2θ=12.7° (±0.2°) and 13.9° (±0.2°). In another embodiment, polymorph Form E can be characterized as having at least one XRPD peak selected from 2θ=6.7° (±0.2°), 9.3° (±0.2°), 12.7° (±0.2°), 13.9° (±0.2°), and 24.4° (±0.2°) in combination with at least one XRPD peak selected from 2θ=12.4° (±0.2°), 13.3° (±0.2°), 14.3° (±0.2°), 15.5° (±0.2°), 17.4° (±0.2°), 18.5° (±0.2°), 22.0° (±0.2°), 23.9° (±0.2°), 24.1° (±0.2°), and 26.4° (±0.2°). In one embodiment, polymorph Form E can be characterized in that it has substantially all of the peaks in its XRPD pattern as shown in FIG. 5.
FIG. 6 shows an XRPD for Polymorph Form F. US8809349
In one embodiment, polymorph Form F can be characterized by any one, two, three, four, five, six, seven, eight, nine, ten, or more of significant peak(s) of FIG. 6. In one embodiment, polymorph Form F can be characterized as having at least one XRPD peak selected from 2θ=9.6° (±0.2°), 17.3° (±0.2°), and 24.6° (±0.2°). In one embodiment, polymorph Form F can be characterized as having at least one XRPD peak selected from 2θ=9.6° (±0.2°), 17.3° (±0.2°), and 24.6° (±0.2°) in combination with at least one XRPD peak selected from 2θ=14.0° (±0.2°) and 19.2° (±0.2°). In another embodiment, polymorph Form F can be characterized as having at least one XRPD peak selected from 2θ=9.6° (±0.2°), 14.0° (±0.2°), 17.3° (±0.2°), 19.2° (±0.2°), and 24.6° (±0.2°) in combination with at least one XRPD peak selected from 2θ=12.4° (±0.2°), 16.1° (±0.2°), 16.6° (±0.2°), 17.1° (±0.2°), 20.8° (±0.2°), 21.5° (±0.2°), 22.0° (±0.2°), 24.3° (±0.2°), 25.2° (±0.2°), and 25.4° (±0.2°). In one embodiment, polymorph Form F can be characterized in that it has substantially all of the peaks in its XRPD pattern as shown in FIG. 6.
FIG. 7 shows an XRPD for Polymorph Form G. US8809349
In one embodiment, polymorph Form G can be characterized by any one, two, three, four, five, six, seven, eight, nine, ten, or more of significant peak(s) of FIG. 7. In one embodiment, polymorph Form G can be characterized as having at least one XRPD peak selected from 2θ=6.7° (±0.2°), 9.5° (±0.2°), and 19.0° (±0.2°). In one embodiment, polymorph Form G can be characterized as having at least one XRPD peak selected from 2θ=6.7° (±0.2°), 9.5° (±0.2°), and 19.0° (±0.2°) in combination with at least one XRPD peak selected from 2θ=10.6° (±0.2°) and 19.6° (±0.2°). In another embodiment, polymorph Form G can be characterized as having at least one XRPD peak selected from 2θ=6.7° (±0.2°), 9.5° (±0.2°), 10.6° (±0.2°), 19.0° (±0.2°), and 19.6° (±0.2°) in combination with at least one XRPD peak selected from 2θ=13.4° (±0.2°), 15.0° (±0.2°), 15.8° (±0.2°), 17.8° (±0.2°), 20.7° (±0.2°), 21.2° (±0.2°), 22.8° (±0.2°), 23.8° (±0.2°), 24.3° (±0.2°), and 25.6° (±0.2°). In one embodiment, polymorph Form G can be characterized in that it has substantially all of the peaks in its XRPD pattern as shown in FIG. 7.
FIG. 8 shows an XRPD for Polymorph Form H. US8809349
In one embodiment, polymorph Form H can be characterized by any one, two, three, four, five, six, seven, eight, nine, ten, or more of significant peak(s) of FIG. 8. In one embodiment, polymorph Form H can be characterized as having at least one XRPD peak selected from 2θ=8.9° (±0.2°), 9.2° (±0.2°), and 14.1° (±0.2°). In one embodiment, polymorph Form H can be characterized as having at least one XRPD peak selected from 2θ=8.9° (±0.2°), 9.2° (±0.2°), and 14.1° (±0.2°) in combination with at least one XRPD peak selected from 2θ=17.3° (±0.2°) and 18.5° (±0.2°). In another embodiment, polymorph Form H can be characterized as having at least one XRPD peak selected from 2θ=8.9° (±0.2°), 9.2° (±0.2°), 14.1° (±0.2°), 17.3° (±0.2°), and 18.5° (±0.2°) in combination with at least one XRPD peak selected from 2θ=7.1° (±0.2°), 10.6° (±0.2°), 11.3° (±0.2°), 11.6° (±0.2°), 16.2° (±0.2°), 18.3° (±0.2°), 18.8° (±0.2°), 20.3° (±0.2°), 21.7° (±0.2°), and 24.7° (±0.2°). In one embodiment, polymorph Form H can be characterized in that it has substantially all of the peaks in its XRPD pattern as shown in FIG. 8.
FIG. 9 shows an XRPD for Polymorph Form I.
In one embodiment, polymorph Form I can be characterized by any one, two, three, four, five, six, seven, eight, nine, ten, or more of significant peak(s) of FIG. 9. In one embodiment, polymorph Form I can be characterized as having at least one XRPD peak selected from 2θ=9.7° (±0.2°), 19.3° (±0.2°), and 24.5° (±0.2°). In one embodiment, polymorph Form I can be characterized as having at least one XRPD peak selected from 2θ=9.7° (±0.2°), 19.3° (±0.2°), and 24.5° (±0.2°) in combination with at least one XRPD peak selected from 2θ=11.4° (±0.2°) and 14.2° (±0.2°). In another embodiment, polymorph Form I can be characterized as having at least one XRPD peak selected from 2θ=9.7° (±0.2°), 11.4° (±0.2°), 14.2° (±0.2°), 19.3° (±0.2°), and 24.5° (±0.2°) in combination with at least one XRPD peak selected from 2θ=9.2° (±0.2°), 14.7° (±0.2°), 15.5° (±0.2°), 16.7° (±0.2°), 17.3° (±0.2°), 18.4° (±0.2°), 21.4° (±0.2°), 22.9° (±0.2°), 29.1° (±0.2°), and 34.1° (±0.2°). In one embodiment, polymorph Form I can be characterized in that it has substantially all of the peaks in its XRPD pattern as shown in FIG. 9.
FIG. 10 shows an XRPD for Polymorph Form J.
In one embodiment, polymorph Form J can be characterized by any one, two, three, four, five, six, seven, eight, nine, ten, or more of significant peak(s) of FIG. 10. In one embodiment, polymorph Form J can be characterized as having at least one XRPD peak selected from 2θ=9.1° (±0.2°), 17.3° (±0.2°), and 18.3° (±0.2°). In one embodiment, polymorph Form J can be characterized as having at least one XRPD peak selected from 2θ=9.1° (±0.2°), 17.3° (±0.2°), and 18.3° (±0.2°) in combination with at least one XRPD peak selected from 2θ=16.4° (±0.2°) and 17.9° (±0.2°). In another embodiment, polymorph Form J can be characterized as having at least one XRPD peak selected from 2θ=9.1° (±0.2°), 16.4° (±0.2°), 17.3° (±0.2°), 17.9° (±0.2°), and 18.3° (±0.2°) in combination with at least one XRPD peak selected from 2θ=9.4° (±0.2°), 10.1° (±0.2°), 10.7° (±0.2°), 14.0° (±0.2°), 14.3° (±0.2°), 15.5° (±0.2°), 16.9° (±0.2°), 19.9° (±0.2°), 24.0° (±0.2°), and 24.7° (±0.2°). In one embodiment, polymorph Form J can be characterized in that it has substantially all of the peaks in its XRPD pattern as shown in FIG. 10.
FIG. 11 shows an XRPD for amorphous compound of Formula (I).
FIG. 12 shows a differential scanning calorimetry (DSC) thermogram for Polymorph Form A.
FIG. 13 shows a DSC for Polymorph Form B.
FIG. 14 shows a DSC for Polymorph Form C.
FIG. 15 shows a DSC for Polymorph Form D.
FIG. 16 shows a DSC for Polymorph Form E.
FIG. 17 shows a DSC for Polymorph Form F.
FIG. 18 shows a DSC for Polymorph Form G.
FIG. 19 shows a DSC for Polymorph Form H.
FIG. 20 shows a DSC for Polymorph Form I.
FIG. 21 shows a DSC for Polymorph Form J.
FIG. 22 shows a DSC thermogram and a thermogravimetric analysis (TGA) for Polymorph Form A.
FIG. 23 shows two DSC thermograms for Polymorph Form C.
FIG. 24 shows a DSC and a TGA for Polymorph Form F.
FIG. 25 shows a panel of salts tested for formation of crystalline solids in various solvents.
FIG. 26 shows a single crystal X-ray structure of Polymorph Form G MTBE (t-butyl methyl ether) solvate of a compound of Formula (I).
FIG. 27 shows an FT-IR spectra of Polymorph Form C.

FIG. 28 shows a 1H-NMR spectra of Polymorph Form C.

FIG. 29 shows a 13C-NMR spectra of Polymorph Form C.

FIG. 30 shows a dynamic vapor sorption (DVS) analysis of Polymorph Form C.
FIG. 31 shows representative dissolution profiles of capsules containing Polymorph Form C.
Enantiomers
Enantiomers can be isolated from racemic mixtures by any method known to those skilled in the art, including chiral high pressure liquid chromatography (HPLC), the formation and crystallization of chiral salts, or prepared by asymmetric syntheses. See, for example, Enantiomers, Racemates and Resolutions (Jacques, Ed., Wiley Interscience, New York, 1981); Wilen et al., Tetrahedron 33:2725 (1977); Stereochemistry of Carbon Compounds (E. L. Eliel, Ed., McGraw-Hill, NY, 1962); and Tables of Resolving Agents and Optical Resolutions p. 268 (E. L. Eliel, Ed., Univ. of Notre Dame Press, Notre Dame, Ind. 1972).
“Tautomer”
The term “tautomer” is a type of isomer that includes two or more interconvertable compounds resulting from at least one formal migration of a hydrogen atom and at least one change in valency (e.g., a single bond to a double bond, a triple bond to a single bond, or vice versa). “Tautomerization” includes prototropic or proton-shift tautomerization, which is considered a subset of acid-base chemistry. “Prototropic tautomerization” or “proton-shift tautomerization” involves the migration of a proton accompanied by changes in bond order. The exact ratio of the tautomers depends on several factors, including temperature, solvent, and pH. Where tautomerization is possible (e.g., in solution), a chemical equilibrium of tautomers can be reached. Tautomerizations (i.e., the reaction providing a tautomeric pair) can be catalyzed by acid or base, or can occur without the action or presence of an external agent. Exemplary tautomerizations include, but are not limited to, keto-to-enol; amide-to-imide; lactam-to-lactim; enamine-to-imine; and enamine-to-(a different) enamine tautomerizations. An example of keto-enol tautomerization is the interconversion of pentane-2,4-dione and 4-hydroxypent-3-en-2-one tautomers. Another example of tautomerization is phenol-keto tautomerization. Another example of phenol-keto tautomerization is the interconversion of pyridin-4-ol and pyridin-4(1H)-one tautomers.
As defined herein, the term “Formula (I)” includes (S)-3-(1-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one in its imide tautomer shown below as (1-1) and in its lactim tautomer shown below as (1-2):
“Polymorph”
“polymorph” can be used herein to describe a crystalline material, e.g., a crystalline form. In certain embodiments, “polymorph” as used herein are also meant to include all crystalline and amorphous forms of a compound or a salt thereof, including, for example, crystalline forms, polymorphs, pseudopolymorphs, solvates, hydrates, co-crystals, unsolvated polymorphs (including anhydrates), conformational polymorphs, tautomeric forms, disordered crystalline forms, and amorphous forms, as well as mixtures thereof, unless a particular crystalline or amorphous form is referred to. Compounds of the present disclosure include crystalline and amorphous forms of those compounds, including, for example, crystalline forms, polymorphs, pseudopolymorphs, solvates, hydrates, co-crystals, unsolvated polymorphs (including anhydrates), conformational polymorphs, tautomeric forms, disordered crystalline forms, and amorphous forms of the compounds or a salt thereof, as well as mixtures thereof.
As used herein, and unless otherwise specified, a particular form of a compound of Formula (I) described herein (e.g., Form A, B, C, D, E, F, G, H, I, J, or amorphous form of a compound of Formula (I), or mixtures thereof) is meant to encompass a solid form of a compound of Formula (I), or a salt, solvate, or hydrate thereof, among others.
As used herein, and unless otherwise specified, a particular form of a compound of Formula (I) described herein (e.g., Form A, B, C, D, E, F, G, H, I, J, or amorphous form of a compound of Formula (I), or mixtures thereof) is meant to encompass a solid form of a compound of Formula (I), or a salt, solvate, or hydrate thereof, among others.
The polymorphs made according to the methods provided herein can be characterized by any methodology known in the art. For example, the polymorphs made according to the methods provided herein can be characterized by X-ray powder diffraction (XRPD), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), dynamic vapor sorption (DVS), hot-stage microscopy, optical microscopy, Karl Fischer analysis, melting point, spectroscopy (e.g., Raman, solid state nuclear magnetic resonance (ssNMR), liquid state nuclear magnetic resonance (1H- and 13C-NMR), and FT-IR), thermal stability, grinding stability, and solubility, among others.
“Solid form”
The terms “solid form” and related terms herein refer to a physical form comprising a compound provided herein or a salt or solvate or hydrate thereof, which is not in a liquid or a gaseous state. Solid forms can be crystalline, amorphous, disordered crystalline, partially crystalline, and/or partially amorphous.
“Crystalline,”
The term “crystalline,” when used to describe a substance, component, or product, means that the substance, component, or product is substantially crystalline as determined, for example, by X-ray diffraction. See, e.g., Remington: The Science and Practice of Pharmacy, Lippincott Williams & Wilkins, 21st ed. (2005).
As used herein, and unless otherwise specified, the term “crystalline form,” “crystal form,” and related terms herein refer to the various crystalline material comprising a given substance, including single-component crystal forms and multiple-component crystal forms, and including, but not limited to, polymorphs, solvates, hydrates, co-crystals and other molecular complexes, as well as salts, solvates of salts, hydrates of salts, other molecular complexes of salts, and polymorphs thereof. In certain embodiments, a crystal form of a substance can be substantially free of amorphous forms and/or other crystal forms. In other embodiments, a crystal form of a substance can contain about 1%, about 2%, about 3%, about 4%, about 5%, about 10%, about 15%, about 20%, about 25%, about 30%, about 35%, about 40%, about 45% or about 50% of one or more amorphous form(s) and/or other crystal form(s) on a weight and/or molar basis.
Certain crystal forms of a substance can be obtained by a number of methods, such as, without limitation, melt recrystallization, melt cooling, solvent recrystallization, recrystallization in confined spaces, such as, e.g., in nanopores or capillaries, recrystallization on surfaces or templates, such as, e.g., on polymers, recrystallization in the presence of additives, such as, e.g., co-crystal counter-molecules, desolvation, dehydration, rapid evaporation, rapid cooling, slow cooling, vapor diffusion, sublimation, grinding, solvent-drop grinding, microwave-induced precipitation, sonication-induced precipitation, laser-induced precipitation, and/or precipitation from a supercritical fluid. As used herein, and unless otherwise specified, the term “isolating” also encompasses purifying.
Characterizing crystal forms and amorphous forms
Techniques for characterizing crystal forms and amorphous forms can include, but are not limited to, thermal gravimetric analysis (TGA), differential scanning calorimetry (DSC), X-ray powder diffractometry (XRPD), single crystal X-ray diffractometry, vibrational spectroscopy, e.g., infrared (IR) and Raman spectroscopy, solid-state nuclear magnetic resonance (NMR) spectroscopy, optical microscopy, hot stage optical microscopy, scanning electron microscopy (SEM), electron crystallography and quantitative analysis, particle size analysis (PSA), surface area analysis, solubility studies, and dissolution studies.
PEAK
As used herein, and unless otherwise specified, the term “peak,” when used in connection with the spectra or data presented in graphical form (e.g., XRPD, IR, Raman, and NMR spectra), refers to a peak or other special feature that one skilled in the art would recognize as not attributable to background noise. The term “significant peak” refers to peaks at least the median size (e.g., height) of other peaks in the spectrum or data, or at least 1.5, 2, or 2.5 times the background level in the spectrum or data.
“Pharmaceutically acceptable carrier”
“pharmaceutically acceptable carrier” or “pharmaceutically acceptable excipient” includes any and all solvents, dispersion media, coatings, antibacterial and antifungal agents, isotonic and absorption delaying agents and the like. The use of such media and agents for pharmaceutically active substances is known in the art. Except insofar as any conventional media or agent is incompatible with the active ingredient, its use in the therapeutic compositions of the present disclosure is contemplated. Supplementary active ingredients can also be incorporated into the compositions.
“Substantially pure”
the term “substantially pure” when used to describe a polymorph, a crystal form, or a solid form of a compound or complex described herein means a solid form of the compound or complex that comprises a particular polymorph and is substantially free of other polymorphic and/or amorphous forms of the compound. A representative substantially pure polymorph comprises greater than about 80% by weight of one polymorphic form of the compound and less than about 20% by weight of other polymorphic and/or amorphous forms of the compound; greater than about 90% by weight of one polymorphic form of the compound and less than about 10% by weight of other polymorphic and/or amorphous forms of the compound; greater than about 95% by weight of one polymorphic form of the compound and less than about 5% by weight of other polymorphic and/or amorphous forms of the compound; greater than about 97% by weight of one polymorphic form of the compound and less than about 3% by weight of other polymorphic and/or amorphous forms of the compound; or greater than about 99% by weight of one polymorphic form of the compound and less than about 1% by weight of other polymorphic and/or amorphous forms of the compound.
“Stable”
The term “stable” refers to a compound or composition that does not readily decompose or change in chemical makeup or physical state. A stable composition or formulation provided herein does not significantly decompose under normal manufacturing or storage conditions. In some embodiments, the term “stable,” when used in connection with a formulation or a dosage form, means that the active ingredient of the formulation or dosage form remains unchanged in chemical makeup or physical state for a specified amount of time and does not significantly degrade or aggregate or become otherwise modified (e.g., as determined, for example, by HPLC, FTIR, or XRPD). In some embodiments, about 70 percent or greater, about 80 percent or greater, about 90 percent or greater, about 95 percent or greater, about 98 percent or greater, or about 99 percent or greater of the compound remains unchanged after the specified period. In one embodiment, a polymorph provided herein is stable upon long-term storage (e.g., no significant change in polymorph form after about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 18, 24, 30, 36, 42, 48, 54, 60, or greater than about 60 months).
Amorphous form
In one embodiment, an amorphous form of a compound of Formula (I), or a pharmaceutically acceptable salt, solvate, or hydrate thereof, can be made by dissolution of a crystalline form followed by removal of solvent under conditions in which stable crystals are not formed. For example, solidification can occur by rapid removal of solvent, by rapid addition of an anti-solvent (causing the amorphous form to precipitate out of solution), or by physical interruption of the crystallization process. Grinding processes can also be used. In other embodiments, an amorphous form of a compound of Formula (I), or a pharmaceutically acceptable salt, solvate, or hydrate thereof, can be made using a process or procedure described herein elsewhere.
In certain embodiments, an amorphous form can be obtained by fast cooling from a single solvent system, such as, e.g., ethanol, isopropyl alcohol, t-amyl alcohol, n-butanol, methanol, acetone, ethyl acetate, or acetic acid. In certain embodiments, an amorphous form can be obtained by slow cooling from a single solvent system, such as, e.g., ethanol, isopropyl alcohol, t-amyl alcohol, or ethyl acetate.
In certain embodiments, an amorphous form can be obtained by fast cooling from a binary solvent system, for example, with acetone or DME as the primary solvent. In certain embodiments, an amorphous form can be obtained by slow cooling from a binary solvent system, for example, with ethanol, isopropyl alcohol, THF, acetone, or methanol as the primary solvent. In some embodiments, an amorphous form can be obtained by dissolution of a compound of Formula (I) in t-butanol and water at elevated temperature, followed by cooling procedures to afford an amorphous solid form.
Salt Forms
In certain embodiments, a compound of Formula (I) provided herein is a pharmaceutically acceptable salt, or a solvate or hydrate thereof. In one embodiment, pharmaceutically acceptable acid addition salts of a compound provided herein can be formed with inorganic acids and organic acids. Inorganic acids from which salts can be derived include, but are not limited to, hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric acid, and the like. Organic acids from which salts can be derived include, but are not limited to, acetic acid, propionic acid, glycolic acid, pyruvic acid, oxalic acid, maleic acid, malonic acid, succinic acid, fumaric acid, tartaric acid, citric acid, benzoic acid, cinnamic acid, mandelic acid, methanesulfonic acid, ethanesulfonic acid, p-toluenesulfonic acid, salicylic acid, and the like. In other embodiments, if applicable, pharmaceutically acceptable base addition salts of a compound provided herein can be formed with inorganic and organic bases. Inorganic bases from which salts can be derived include, but are not limited to, sodium, potassium, lithium, ammonium, calcium, magnesium, iron, zinc, copper, manganese, aluminum, and the like. Organic bases from which salts can be derived include, but are not limited to, primary, secondary, and tertiary amines, substituted amines including naturally occurring substituted amines, cyclic amines, basic ion exchange resins, and the like. Exemplary bases include, but are not limited to, isopropylamine, trimethylamine, diethylamine, triethylamine, tripropylamine, and ethanolamine. In some embodiments, a pharmaceutically acceptable base addition salt is ammonium, potassium, sodium, calcium, or magnesium salt. In one embodiment, bis salts (i.e., two counterions) and higher salts (e.g., three or more counterions) are encompassed within the meaning of pharmaceutically acceptable salts.
In certain embodiments, salts of a compound of Formula (I) can be formed with, e.g., L-tartaric acid, p-toluenesulfonic acid, D-glucaronic acid, ethane-1,2-disulfonic acid (EDSA), 2-naphthalenesulfonic acid (NSA), hydrochloric acid (HCl) (mono and bis), hydrobromic acid (HBr), citric acid, naphthalene-1,5-disulfonic acid (NDSA), DL-mandelic acid, fumaric acid, sulfuric acid, maleic acid, methanesulfonic acid (MSA), benzenesulfonic acid (BSA), ethanesulfonic acid (ESA), L-malic acid, phosphoric acid, and aminoethanesulfonic acid (taurine).
(R)- and (S)-isomers
In some embodiments, the (R)- and (S)-isomers of the non-limiting exemplary compounds, if present, can be resolved by methods known to those skilled in the art, for example by formation of diastereoisomeric salts or complexes which can be separated, for example, by crystallization; via formation of diastereoisomeric derivatives which can be separated, for example, by crystallization, gas-liquid or liquid chromatography; selective reaction of one enantiomer with an enantiomer-specific reagent, for example enzymatic oxidation or reduction, followed by separation of the modified and unmodified enantiomers; or gas-liquid or liquid chromatography in a chiral environment, for example on a chiral support, such as silica with a bound chiral ligand or in the presence of a chiral solvent. Alternatively, a specific enantiomer can be synthesized by asymmetric synthesis using optically active reagents, substrates, catalysts or solvents, or by converting one enantiomer to the other by asymmetric transformation.
XRPD
Compounds and polymorphs provided herein can be characterized by X-ray powder diffraction patterns (XRPD). The relative intensities of XRPD peaks can vary depending upon the sample preparation technique, the sample mounting procedure and the particular instrument employed, among other parameters. Moreover, instrument variation and other factors can affect the 2θ peak values. Therefore, in certain embodiments, the XRPD peak assignments can vary by plus or minus about 0.2 degrees theta or more, herein referred to as “(±0.2°)”.
XRPD patterns for each of Forms A-J and amorphous form of the compound of Formula (I) were collected with a PANalytical CubiX XPert PRO MPD diffractometer using an incident beam of CU radiation produced using an Optix long, fine-focus source. An elliptically graded multilayer mirror was used to focus Cu Kα X-rays through the specimen and onto the detector. Samples were placed on Si zero-return ultra-micro sample holders. Analysis was performed using a 10 mm irradiated width and the following parameters were set within the hardware/software:
| X-ray tube: | Cu Kα, 45 kV, 40 mA | ||
| Detector: | X′Celerator | ||
| Slits: | ASS Primary Slit: Fixed 1° | ||
| Divergence Slit (Prog): | Automatic - 5 mm irradiated length | ||
| Soller Slits: | 0.02 radian | ||
| Scatter Slit (PASS): | Automatic - 5 mm observed length | ||
| Scanning | |||
| Scan Range: | 3.0-45.0° | ||
| Scan Mode: | Continuous | ||
| Step Size: | 0.03° | ||
| Time per Step: | 10 s | ||
| Active Length: | 2.54° | ||
DSC
Compounds and polymorphs provided herein can be characterized by a characteristic differential scanning calorimeter (DSC) thermogram. For DSC, it is known in the art that the peak temperatures observed will depend upon the rate of temperature change, the sample preparation technique, and the particular instrument employed, among other parameters. Thus, the peak values in the DSC thermograms reported herein can vary by plus or minus about 2° C., plus or minus about 3° C., plus or minus about 4° C., plus or minus about 5° C., plus or minus about 6° C., to plus or minus about 7° C., or more. For some polymorph Forms, DSC analysis was performed on more than one sample which illustrates the known variability in peak position, for example, due to the factors mentioned above. The observed peak positional differences are in keeping with expectation by those skilled in the art as indicative of different samples of a single polymorph Form of a compound of Formula (I).
Impurities in a sample can also affect the peaks observed in any given DSC thermogram. In some embodiments, one or more chemical entities that are not the polymorph of a compound of Formula (I) in a sample being analyzed by DSC can result in one or more peaks at lower temperature than peak(s) associated with the transition temperature of a given polymorph as disclosed herein.
DSC analyses were performed using a Mettler 822e differential scanning calorimeter. Samples were weighed in an aluminum pan, covered with a pierced lid, and then crimped. General analysis conditions were about 30° C. to about 300° C.-about 350° C. ramped at about 10° C./min. Several additional ramp rates were utilized as part of the investigation into the high melt Form B, including about 2° C./min, about 5° C./min, and about 20° C./min. Samples were analyzed at multiple ramp rates to measure thermal and kinetic transitions observed.
Isothermal holding experiments were also performed utilizing the DSC. Samples were ramped at about 10° C./min to temperature (about 100° C. to about 250° C.) and held for about five minutes at temperature before rapid cooling to room temperature. In these cases, samples were then analyzed by XRPD or reanalyzed by DSC analysis.
TGA
A polymorphic form provided herein can give rise to thermal behavior different from that of an amorphous material or another polymorphic form. Thermal behavior can be measured in the laboratory by thermogravimetric analysis (TGA) which can be used to distinguish some polymorphic forms from others. In one embodiment, a polymorph as disclosed herein can be characterized by thermogravimetric analysis.
TGA analyses were performed using a Mettler 851e SDTA/TGA thermal gravimetric analyzer. Samples were weighed in an alumina crucible and analyzed from about 30° C. to about 230° C. and at a ramp rate of about 10° C./min.
DVS
Compounds and polymorphs provided herein can be characterized by moisture sorption analysis. This analysis was performed using a Hiden IGAsorp Moisture Sorption instrument. Moisture sorption experiments were carried out at about 25° C. by performing an adsorption scan from about 40% to about 90% RH in steps of about 10% RH and a desorption scan from about 85% to about 0% RH in steps of about −10% RH. A second adsorption scan from about 10% to about 40% RH was performed to determine the moisture uptake from a drying state to the starting humidity. Samples were allowed to equilibrate for about four hours at each point or until an asymptotic weight was reached. After the isothermal sorption scan, samples were dried for about one hour at elevated temperature (about 60° C.) to obtain the dry weight. XRPD analysis on the material following moisture sorption was performed to determine the solid form.
Optical Microscopy
Compounds and polymorphs provided herein can be characterized by microscopy, such as optical microscopy. Optical microscopy analysis was performed using a Leica DMRB Polarized Microscope. Samples were examined with a polarized light microscope combined with a digital camera (1600×1200 resolution). Small amounts of samples were dispersed in mineral oil on a glass slide with cover slips and viewed with 100× magnification.
Karl Fischer Analysis
Compounds and polymorphs provided herein can be characterized by Karl Fischer analysis to determine water content. Karl Fischer analysis was performed using a Metrohm 756 KF Coulometer. Karl Fisher titration was performed by adding sufficient material to obtain 50 μg of water, about 10 to about 50 mg of sample, to AD coulomat.
Raman Spectroscopy
Compounds and polymorphs provided herein can be characterized by Raman spectroscopy. Raman spectroscopy analysis was performed using a Kaiser RamanRXN1 instrument with the samples in a glass well. Raman spectra were collected using a PhAT macroscope at about 785 nm irradiation frequency and about 1.2 mm spot size. Samples were analyzed using 12 to 16 accumulations with about 0.5 to about 12 second exposure time and utilized cosmic ray filtering. The data was processed by background subtraction of an empty well collected with the same conditions. A baseline correction and smoothing was performed to obtain interpretable data when necessary.
FT-IR
Compounds and polymorphs provided herein can be characterized by FT-IR spectroscopy. FT-IR spectroscopy was performed using either a Nicolet Nexus 470 or Avatar 370 Infrared Spectrometer and the OMNIC software. Samples were analyzed using a diamond Attenuated Total Reflection (ATR) accessory. A compound sample was applied to the diamond crystal surface and the ATR knob was turned to apply the appropriate pressure. The spectrum was then acquired and analyzed using the OMNIC software. Alternative sample preparations include solution cells, mulls, thin films, and pressed discs, such as those made of KBr, as known in the art.
NMR
Compounds and polymorphs provided herein can be characterized by nuclear magnetic resonance (NMR). NMR spectra were obtained using a 500 MHz Bruker AVANCE with 5-mm BBO probe instrument. Samples (approximately 2 to approximately 10 mg) were dissolved in DMSO-d6 with 0.05% tetramethylsilane (TMS) for internal reference. 1H-NMR spectra were acquired at 500 MHz using 5 mm broadband observe (1H-X) Z gradient probe. A 30 degree pulse with 20 ppm spectral width, 1.0 s repetition rate, and 32-64 transients were utilized in acquiring the spectra.
High-Performance Liquid Chromatography
Compounds and polymorphs provided herein can be analyzed by high-performance liquid chromatography using an Agilent 1100 instrument. The instrument parameters for achiral HPLC are as follows:
| Column: | Sunfire C18 4.6 × 150 mm | ||
| Column Temperature: | Ambient | ||
| Auto-sampler Temperature: | Ambient | ||
| Detection: | UV at 250 nm | ||
| Mobile Phase A: | 0.05% trifluoroacetic acid in water | ||
| Mobile Phase B: | 0.05% trifluoroacetic acid in MeCN | ||
| Flow Rate: | 1.0 mL/minute | ||
| Injection Volume: | 10 μL | ||
| Data Collection time: | 20 minutes | ||
| Re-equilibration Time: | 5 minutes | ||
| Diluent & Needle Wash: | MeOH | ||
Gradient Conditions:
| Time (minutes) | % A | % B |
| 0.0 | 90 | 10 |
| 3.5 | 90 | 10 |
| 10.0 | 10 | 90 |
| 15.0 | 10 | 90 |
| 18.0 | 90 | 10 |
| 20.0 | 90 | 10 |
Compounds and polymorphs provided herein can be analyzed by high-performance liquid chromatography using a chiral HPLC column to determine % ee values:
| Column: | Chiralpak IC, 4.6 mm × 250 mm, 5 μm. |
| Column Temperature: | Room Temperature |
| Sample Temperature: | Room Temperature |
| Detection: | UV at 254 nm |
| Mobile Phase A: | 60% Hexane 40% (IPA: EtOH = 2:3) with 0.2% |
| Acetic Acid and 0.1% DEA | |
| Isocratic: | 100% A |
| Flow Rate: | 1 mL/min |
| Diluent: | Methanol |
| Injection Volume: | 10 μL |
| Analysis Time: | 25 min |
Example 8
Analytical Data of (S)-3-(1-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one
Provided herein are analytical data of various purified samples of (S)-3-(1-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one, the compound of Formula (I). Confirmation of the structure of the compound of Formula (I) was obtained via single crystal X-ray diffraction, FT-IR, 1H-NMR and 13C-NMR spectra.
A single crystal structure of a tert-butyl methyl ether solvate of (S)-3-(1-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one (e.g., polymorph Form G) was generated and single crystal X-ray data was collected. The structure is shown in FIG. 26, which further confirmed the absolute stereochemistry as the S-enantiomer.
FT-IR spectra of Form C of (S)-3-(1-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one was obtained, and shown in FIG. 27.
1H-NMR and 13C-NMR spectra of a sample of Form C of (S)-3-(1-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one were obtained, and are provided in FIG. 28 and FIG. 29, respectively.
Example 9
General Methods for the Preparation of Polymorphs Form A, B, C, D, E, F, G, H, I, J of the Compound of Formula (I)
General Method A: Single Solvent Crystallization with Fast Cooling or Slow Cooling
A sample of a compound of Formula (I) (e.g., Form A or Form C) is placed into a vial equipped with stir bar and dissolved with a minimal amount of solvent (such as about 0.2 mL to about 0.3 mL) at an elevated temperature. The resulting solution is polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, the vial is placed in a refrigerator (e.g., about 4° C.) overnight in a fast cooling procedure, or cooled to ambient temperature at a rate of about 20° C./h and allowed to equilibrate without stiffing at ambient temperature overnight in a slow cooling procedure. Optionally, a sample without solids can be scratched with an implement known in the art (e.g., a spatula) to initiate crystallization. The solution can be allowed to equilibrate for a period of time, such as approximately 8 hours. For a slow cooling sample, if scratching does not provide solids after about 8 hours, then a stir bar can be added and the sample then stirred overnight. A sample without precipitation can be evaporated to dryness under a gentle gas stream, such as argon, nitrogen, ambient air, etc. The precipitated solids can be recovered by vacuum filtration, centrifuge filtration, or decanted as appropriate to afford the Form as indicated below.
General Method B: Multi-Solvent Crystallization with Fast Cooling or Slow Cooling
Multi-solvent (e.g., binary) solvent crystallizations can be performed. Primary solvents include, but are not limited to, ethanol, isopropyl alcohol, methanol, tetrahydrofuran, acetone, methyl ethyl ketone, dioxane, NMP, DME, and DMF. Anti-solvents include, but are not limited to, MTBE, DCM, toluene, heptane, and water.
A sample of a compound of Formula (I) (e.g., Form A or Form C) is placed into a vial equipped with stir bar and dissolved with a minimal amount of solvent (such as about 0.2 mL to about 0.3 mL) at an elevated temperature. The resulting solution is polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, the anti-solvent is added until turbidity is observed. After hot filtration, the vial is placed in a refrigerator (e.g., about 4° C.) overnight in a fast cooling procedure, or cooled to ambient temperature at a rate of about 20° C./h and allowed to equilibrate without stirring at ambient temperature overnight in a slow cooling procedure. Optionally, a sample without solids can be scratched with an implement known in the art (e.g., a spatula) to initiate crystallization. The solution can be allowed to equilibrate for a period of time, such as approximately 8 hours. For a slow cooling sample, if scratching does not provide solids after about 8 hours, then a stir bar can be added and the sample then stirred overnight. A sample without precipitation can be evaporated to dryness under a gentle gas stream, such as argon, nitrogen, ambient air, etc. The precipitated solids can be recovered by vacuum filtration, centrifuge filtration, or decanted as appropriate to afford the Form as indicated below.
General Method C: Slurry Procedures to Afford Formula (I) Polymorph Forms
A mixture of one or more Forms (e.g., Form A or Form C) of the compound of Formula (I) are placed in a vial equipped with a stir bar. A minimal amount of solvent (e.g., a single solvent or a mixture/solution of two or more solvents) is added to the vial to form a heterogeneous slurry. Optionally, the vial can be sealed to prevent evaporation. The slurry is stirred for a period of time ranging from less than about an hour, to about 6 hours, to about 12 hours, to about 24 hours, to about 2 days, to about 4 days, to about 1 week, to about 1.5 weeks, to about 2 weeks or longer. Aliquots can be taken during the stirring period to assess the Form of the solids using, for example, XRPD analysis. Optionally, additional solvent(s) can be added during the stirring period. Optionally, seeds of a given polymorph Form of the compound of Formula (I) can be added. In some cases, the slurry is then stirred for a further period of time, ranging as recited above. The recovered solids can be recovered by vacuum filtration, centrifuge filtration, or decanted as appropriate to afford the Form as indicated below.
Example 10
Preparation of Polymorphs Form A, B, C, D, E, F, G, H, I, J of the Compound of Formula (I)
Form A
Single Solvent Crystallizations to Afford Formula (I) Form A
1. Fast Cooling Procedure From MeCN: Approximately 23 mg of Formula (I) Form A was placed into a 20-mL glass vial equipped with a stir bar. To the vial was added a minimal amount of acetonitrile (7.4 ml) to just dissolve the solids at 70° C. The resulting solution was polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, the vial was placed in a refrigerator (4° C.) overnight. Once at 4° C., the contents of the vial were periodically scratched with a spatula to induce crystallization, and then allowed to equilibrate for approximately 8 hours. The crystals were collected by decanting off the liquid and dried under vacuum (30 inches Hg) at ambient temperature overnight. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
2. Slow Cooling Procedure From MeCN: Approximately 24 mg of Formula (I) Form A was placed into a 20-mL glass vial equipped with a stir bar. To the vial was added a minimal amount of acetonitrile (8 ml) to just dissolve the solids at 70° C. The resulting solution was polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, the vial was cooled to ambient temperature at a rate of 20° C./h and allowed to equilibrate without stirring at ambient temperature overnight. After the equilibration hold at ambient temperature, the contents of the vial were periodically scratched with a spatula to induce crystallization, and then allowed to equilibrate for approximately 8 hours. The crystals were collected by decanting off the liquids and dried under vacuum (30 inches Hg) at ambient temperature overnight. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
3. Slow Cooling Procedure From n-Butanol: Approximately 23 mg of Formula (I) Form A was placed into a 2-dram glass vial equipped with a stir bar. To the vial was added a minimal amount of n-butanol (0.6 ml) to just dissolve the solids at 70° C. The resulting solution was polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, the vials were cooled to ambient temperature at a rate of 20° C./h and allowed to equilibrate without stirring at ambient temperature overnight. After the equilibration hold at ambient temperature, the contents of the vial were periodically scratched with a spatula to induce crystallization, and then allowed to equilibrate for approximately 8 hours. To further induce crystallization, a stir bar was added to the vial and the contents stirred overnight. The resulting crystals were collected by filtration and dried under vacuum (30 inches Hg) at ambient temperature overnight. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
Binary Solvent Crystallizations to Afford Formula (I) Form A
1. Fast Cooling Procedure From Acetone/DCM: Approximately 23.5 mg of Formula (I) Form A was placed into a 2-dram glass vial equipped with a stir bar. To the vial was added a minimal amount of acetone (2.6 ml) to just dissolve the solids at 50° C. The resulting solution was polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, DCM (5.0 ml) was added portion-wise. After the anti-solvent addition, the vials were placed in a refrigerator (4° C.) overnight. Once at 4° C., the contents of the vial were periodically scratched with a spatula to induce crystallization, and then allowed to equilibrate for approximately 8 hours. The crystals were collected by filtration and dried under vacuum (30 inches Hg) at ambient temperature overnight. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
2. Fast Cooling Procedure From MEK/DCM: Approximately 23 mg of Formula (I) Form A was placed into a 2-dram glass vial equipped with a stir bar. To the vial was added a minimal amount of MEK (2.2 ml) to just dissolve the solids at 70° C. The resulting solution was polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, DCM (5.0 ml) was added portion-wise. After the anti-solvent addition, the vial was placed in a refrigerator (4° C.) overnight. Once at 4° C., the contents of the vial were periodically scratched with a spatula to induce crystallization, and then allowed to equilibrate for approximately 8 hours. The crystals were collected by filtration and dried under vacuum (30 inches Hg) at ambient temperature overnight. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
3. Fast Cooling Procedure From DMF/DCM: Approximately 24 mg of Formula (I) Form A was placed into a 2-dram glass vial equipped with a stir bar. To the vial was added a minimal amount of DCM (0.2 ml) to just dissolve the solids at 70° C. The resulting solution was polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, DCM (7.0 ml) was added portion-wise. After the anti-solvent addition, the vial was placed in a refrigerator (4° C.) overnight. Once at 4° C., the contents of the vial were periodically scratched with a spatula to induce crystallization, and then allowed to equilibrate for approximately 8 hours. The crystals were collected by filtration and dried under vacuum (30 inches Hg) at ambient temperature overnight. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
4. Fast Cooling Procedure From Dioxane/DCM: Approximately 24.4 mg of Formula (I) Form A was placed into a 2-dram glass vial equipped with a stir bar. To the vial was added a minimal amount of dioxane (0.8 ml) to just dissolve the solids at 70° C. The resulting solution was polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, DCM (7.0 ml) was added portion-wise. After the anti-solvent addition, the vial was placed in a refrigerator (4° C.) overnight. Once at 4° C., the contents of the vial were periodically scratched with a spatula to induce crystallization, and then allowed to equilibrate for approximately 8 hours. The crystals were collected by filtration and dried under vacuum (30 inches Hg) at ambient temperature overnight. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
5. Slow Cooling Procedure From Acetone/DCM: Approximately 22 mg of Formula (I) Form A was placed into a 2-dram glass vial equipped with a stir bar. To the vial was added a minimal amount of acetone (2.5 ml) to just dissolve the solids at 50° C. The resulting solution was polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, DCM (5.0 ml) was added portion-wise. After the anti-solvent addition, the vial was cooled to ambient temperature at a rate of 20° C./h and allowed to equilibrate without stirring at ambient temperature overnight. After the equilibration hold at ambient temperature, the contents of the vial were periodically scratched with a spatula to induce crystallization, and then allowed to equilibrate for approximately 8 hours. To further induce crystallization, a stir bar was added to the vial and the contents stirred overnight. The resulting crystals were collected by filtration and dried under vacuum (30 inches Hg) at ambient temperature overnight. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
6. Slow Cooling Procedure From MEK/DCM: Approximately 23.4 mg of Formula (I) Form A was placed into a 2-dram glass vial equipped with a stir bar. To the vial was added a minimal amount of MEK (2.2 ml) to just dissolve the solids at 70° C. The resulting solution was polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, DCM (5.0 ml) was added portion-wise. After the anti-solvent addition, the vial was cooled to ambient temperature at a rate of 20° C./h and allowed to equilibrate without stirring at ambient temperature overnight. After the equilibration hold at ambient temperature, the contents of the vial were periodically scratched with a spatula to induce crystallization, and then allowed to equilibrate for approximately 8 hours. The resulting crystals were collected by filtration and dried under vacuum (30 inches Hg) at ambient temperature overnight. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
7. Slow Cooling Procedure From Dioxane/DCM: Approximately 24 mg of Formula (I) Form A was placed into a 2-dram glass vial equipped with a stir bar. To the vial was added a minimal amount of dioxane (0.8 ml) to just dissolve the solids at 70° C. The resulting solution was polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, DCM (7.0 ml) was added portion-wise. After the anti-solvent addition, the vial was cooled to ambient temperature at a rate of 20° C./h and allowed to equilibrate without stirring at ambient temperature overnight. After the equilibration hold at ambient temperature, the contents of the vial were periodically scratched with a spatula to induce crystallization, and then allowed to equilibrate for approximately 8 hours. To further induce crystallization, a stir bar was added to the vial and the contents stirred overnight. The resulting crystals were collected by filtration and dried under vacuum (30 inches Hg) at ambient temperature overnight. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
8. Slow Cooling Procedure From DMF/DCM: Approximately 23.5 mg of Formula (I) Form A was placed into a 2-dram glass vial equipped with a stir bar. To the vial was added a minimal amount of DMF (0.2 ml) to just dissolve the solids at 70° C. The resulting solution was polish filtered through a 0.45 μm syringe filter into a clean preheated vial. After hot filtration, DCM (7.0 ml) was added portion-wise. After the anti-solvent addition, the vial was cooled to ambient temperature at a rate of 20° C./h and allowed to equilibrate without stirring at ambient temperature overnight. After the equilibration hold at ambient temperature, the contents of the vial were periodically scratched with a spatula to induce crystallization, and then allowed to equilibrate for approximately 8 hours. To further induce crystallization, a stir bar was added to the vial and the contents stirred overnight. To further induce crystallization, the contents of the vial were concentrated under a gentle stream of nitrogen to near dryness. The resulting crystals were collected by filtration and dried under vacuum (30 inches Hg) at ambient temperature overnight. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
Slurry Procedure to Afford Formula (I) Form A
1. Procedure from CH2Cl2 and from IPA: Form C (1 g) was slurried in five volumes of dichloromethane. After holding for 15 hours, filtration, and drying, Form A was isolated in 82% yield. Scale-up was performed on a 20 g scale with a water-wet cake of Form C to yield Form A in 92% yield. Drying at 70° C. for six days indicated no degradation in chemical or chiral purity. Slurrying dry Form C in isopropyl alcohol using a similar method also yielded Form A.
2. Procedure for Competitive Slurry Experiment (using forms A, B and C): Competitive slurries were performed by charging approximately a 50/50 mixture of Forms A and C (11.2 mg of Form A and 11.7 mg Form C) to a 1-dram glass vial equipped with a glass stir bar. To the vial was added 600 μL of MeCN. The vial cap was wrapped with parafilm to prevent evaporation. The slurry was stirred for 1 day and an aliquot was taken. The contents of the vial were allowed to stir for an additional week and another aliquot was taken. Both aliquots were centrifuge filtered for five minutes at 8000 RPM. XRPD analysis was performed on the solids from each aliquot to show that the Formula (I) had converted to Form A at both time points. After the one week aliquot was taken, an additional 300 μL of acetonitrile was added to the remaining slurry and allowed to equilibrate for one day. The slurry was then seeded with approximately 3.2 mg of Form B and allowed to equilibrate for an additional three days. The solids were isolated by centrifuge filtration (5 minutes at 8000 RPM) and dried over night under vacuum. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
3. Procedure for Competitive Slurry Experiment (using forms A, C, D, and E): Competitive slurries were performed by charging an approximately equal mixture of each form (7.8 mg of Form A, 7.7 mg Form C, 7.7 mg of Form D, and 8.2 mg of Form E) to a 1-dram glass vial equipped with a glass stir bar. To the vial was added 1 ml of 2-propanol. The vial cap was wrapped with parafilm to prevent evaporation. The slurry was mixed for 1 day and an aliquot was taken. The contents of the vial were allowed to stir for an additional week and another aliquot was taken. Both aliquots were centrifuge filtered for five minutes at 8000 RPM. XRPD analysis was performed on the solids from each aliquot to show that the Formula (I) had converted to Form A at both time points. After the one week aliquot was taken, the remaining solids were isolated by centrifuge filtration (5 minutes at 8000 RPM) and dried over night under vacuum. The dried solids were evaluated for crystallinity and form by XRPD which indicated the crystalline material was polymorph Form A.
Using the General Method B of Example 9, the following experiments detailed in Tables 4 and 5 were performed to afford Formula (I) Form C. Table 4 experiments were conducted using the fast cooling procedure, while Table 5 experiments were conducted using the slow cooling procedure.
Table 4. Fast Cooling Procedure
Table 5. Slow Cooling Procedure
Using General Method C of Example 9, the following experiments detailed in Table 6 were performed to afford the polymorph Form of the compound of Formula (I) as indicated.
Table 6:
Example 12
XRPD Studies
[00653] Using the XRPD instrument and parameters described above, the following XRPD peaks were observed for Formula (I) Polymorph Forms A, B, C, D. E, F, G, H, I, and J. The XRPD traces for these ten polymorph forms are given in Figures 1-10, respectively. In Table 7, peak position units are °2Θ. In one embodiment, a given polymorph Form can be characterized as having at least one of the five XRPD peaks given in Set 1 in Table 7. In another embodiment, the given Form can be characterized as having at least one of the five XRPD peaks given in Set 1 in combination with at least one of the XRPD peaks given in Set 2 in Table 7. In some embodiments, one or more peak position values can be defined as being modified by the term "about" as described herein. In other embodiments, any given peak position is with ±0.2 2Θ (e.g., 9.6+0.2 2Θ).
Table 7.
Example 13
Differential Scanning Calorimetry (DSC) Studies
[00654] Using the DSC instrument and parameters described above, the following DSC peaks were observed for the compound of Formula (I) polymorph Forms A, B, C, D. E, F, G, H, I, and J. The DSC thermograms for these nine polymorph forms are given in FIGS. 12-24, respectively, and peak positions are given in Table 8. Further DSC data for Polymorph Forms A, B, C, D. E, F, G, H, I, and J is given in Table 9 below. Unless marked with a Λ that indicates an exothermic peak, all peaks are endothermic.
Table 8.
Table 9 summarizes non-limiting exemplary preparation techniques for Formula (I) Polymorph Forms A-J and representative analytical data as described below and elsewhere.
Table 9.
Extras.......
SEE NMR ..........http://www.medkoo.com/Product-Data/IPI-145/IPI-145-QC-SSC20130422Web.pdf