

Zidebactam, WCK 5107
Wockhardt Limited

Useful for treating bacterial infections
CAS 1436861-97-0, UNII: YPM97423DB,
Wockhardt Biopharm
Molecular Formula, C13-H21-N5-O7-S
Molecular Weight, 391.4029
Disclosed in PCT International Patent Application No. PCT/IB2012/054290D
- 01 Aug 2015 Phase-I clinical trials in Bacterial infections (In volunteers, Combination therapy) in USA (IV) (NCT02532140)
trans- sulphuric acid mono-[2-(N’-[(R)-piperidin-3-carbonyl]-hydrazinocarbonyl)-7-oxo-l,6-diaza-bicyclo[3.2.1]oct-6-yl] ester
(2S, 5R)-sulphuric acid mono-[2-(N’-[(R)-piperidin-3-carbonyl]-hydrazinocarbonyl)-7-oxo-l,6-diaza-bicyclo[3.2.1]oct-6-yl] ester
(1R,2S,5R)-l,6-Diazabicyclo [3.2.1] octane-2-carboxylic acid, 7-oxo-6-(sulfooxy)-, 2-[2-[(3R)-3-piperidinylcarbonyl]hydrazide]
trans- sulphuric acid mono-[2-(N’-[(R)-piperidin-3-carbonyl]-hydrazinocarbonyl)-7-oxo-l,6-diaza-bicyclo[3.2.1]oct-6-yl] ester
(2S, 5R)-sulphuric acid mono-[2-(N’-[(R)-piperidin-3-carbonyl]-hydrazinocarbonyl)-7-oxo-l,6-diaza-bicyclo[3.2.1]oct-6-yl] ester
(lR,2S,5R)-l,6-Diazabicyclo [3.2.1] octane-2-carboxylic acid, 7-oxo-6-(sulfooxy)-, 2-[2-[(3R)-3 -piperidinylcarbonyl] hydrazide]
1,6-Diazabicyclo(3.2.1)octane-2-carboxylic acid, 7-oxo-6-(sulfooxy)-, 2-(2-((3R)-3-piperidinylcarbonyl)hydrazide), (1R,2S,5R)-
 Zidebactam potassium
cas is 1706777-49-2
Zidebactam sodium ………..below
Zidebactam potassium
cas is 1706777-49-2
Zidebactam sodium ………..below

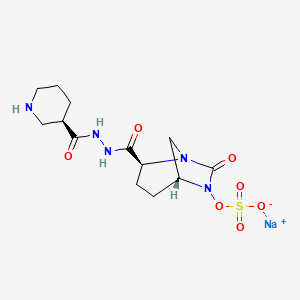
Cas 1706777-46-9
Sodium;[(2S,5R)-7-oxo-2-[[[(3R)-piperidine-3-carbonyl]amino]carbamoyl]-1,6-diazabicyclo[3.2.1]octan-6-yl] sulfate
UNII-NHY7N0Y9DG; NHY7N0Y9DG; Zidebactam sodium; Zidebactam sodium,
(-)-; 1,6-Diazabicyclo(3.2.1)octane-2-carboxylic acid,
7-oxo-6-(sulfooxy)-, 2-(2-((3R)-3-piperidinylcarbonyl)hydrazide), sodium
salt (1:1), (1R,2S,5R)-; 1706777-46-9;
| Molecular Formula: |
C13H20N5NaO7S |
| Molecular Weight: |
413.381969 g/mol |
In September 2015, the drug was reported to be in phase I clinical
trial.One of the family members US09132133, claims a combination of
sulbactam and WCK-5107.
Bacterial infections continue to remain one of the major causes
contributing towards human diseases. One of the key challenges in
treatment of bacterial infections is the ability of bacteria to develop
resistance to one or more antibacterial agents over time. Examples of
such bacteria that have developed resistance to typical antibacterial
agents include: Penicillin-resistant Streptococcus pneumoniae,
Vancomycin-resistant Enterococci, and Methicillin-resistant
Staphylococcus aureus. The problem of emerging drug-resistance in
bacteria is often tackled by switching to newer antibacterial agents,
which can be more expensive and sometimes more toxic. Additionally, this
may not be a permanent solution as the bacteria often develop
resistance to the newer antibacterial agents as well in due course. In
general, bacteria are particularly efficient in developing resistance,
because of their ability to multiply very rapidly and pass on the
resistance genes as they replicate.
Treatment of infections caused by resistant bacteria remains a key
challenge for the clinician community. One example of such challenging
pathogen is Acinetobacter baumannii (A. baumannii), which continues to
be an increasingly important and demanding species in healthcare
settings. The multidrug resistant nature of this pathogen and its
unpredictable susceptibility patterns make empirical and therapeutic
decisions more difficult. A. baumannii is associated with infections
such as pneumonia, bacteremia, wound infections, urinary tract
infections and meningitis.
Therefore, there is a need for development of newer ways to treat
infections that are becoming resistant to known therapies and methods.
Surprisingly, it has been found that a compositions comprising cefepime
and certain nitrogen containing bicyclic compounds (disclosed in
PCT/IB2012/054290) exhibit unexpectedly synergistic antibacterial
activity, even against highly resistant bacterial strains.


PATENT
http://www.google.com/patents/WO2013030733A1?cl=en
Scheme-1
function with Boc group)
o ormua –
Scheme-2
Example-2 trans-sulfuric acid mono-r2-(N
,-r(R)-piperidin-3-carbonyll-hvdrazinocarbonyl)-7-oxo-l,6- diaza-bicyclo Γ3.2.11 oct-6-νΠ ester
Step-1: Preparation of
trans-3-[N’-(6-benzyloxy-7-oxo-l,6-diaza-bicyclo[3.2.1]octane-2-
carbonyl)-hydrazinocarbonyl]-(R)-piperidin-l-carboxylic acid tert-butyl
ester:
By using the procedure described in Step-1 of Example- 1 above, and
by using trans-6-
benzyloxy-7-oxo-l,6-diaza-bicyclo[3.2.1]octane-2-carboxylic acid (25 gm,
0.084 mol), N,N- dimethyl formamide (625 ml), EDC hydrochloride (24 gm,
0.126 mol), HOBt (16.96 gm, 0.126 mol),
(R)-N-tert-butoxycarbonyl-piperidin-3-carboxylic acid hydrazide (21.40
gm , 0.088 mol) to provide the title compound in 17.0 gm quantity, 41%
yield as a white solid.
Analysis: MS (ES+) CzsHasNsOe = 502.1 (M+l);
I^NMR (CDCI
3) = 8.40 (br s, IH), 7.34-7.44 (m, 5H), 5.05
(d, IH), 4.90 (d, IH), 4.00 (br d, IH), 3.82 (br s, IH), 3.30 (br s,
IH), 3.16-3.21 (m, IH), 3.06 (br d, IH), 2.42 (br s, IH), 2.29-2.34 (m,
IH), 1.18-2.02 (m, 4H), 1.60-1.75 (m, 4H), 1.45-1.55 (m, 2H),1.44 (s,
9H).
Step-2: Preparation of
trans-3-[N’-(6-hydroxy-7-oxo-l,6-diaza-bicyclo[3.2.1]octane-2-
carbonyl)-hydrazinocarbonyl]-(R)-piperidin-l-carboxylic acid tert-butyl
ester:
By using the procedure described in Step-2 of Example- 1 above, and
by using trans-3- [N ‘ -(6-benzyloxy-7-oxo- 1 ,6-diaza-bicyclo [3.2.1
]octane-2-carbonyl)-hydrazinocarbonyl] -(R)- piperidin-l-carboxylic acid
tert-butyl ester (16.5 gm , 0.033 mol), methanol (170 ml) and 10%
palladium on carbon (3.5 gm) to provide the title compound in 13.5 gm
quantity as a pale pink solid and it was used for the next reaction
immediately.
Analysis: MS (ES+) CiglfeNsOe = 411.1 (M+l);
Step-3: Preparation of tetrabutylammonium salt of
trans-3-[N’-(6-sulfooxy-7-oxo-l,6-diaza- bicyclo [3.2.1]
octane-2-carbonyl)-hydrazinocarbonyl] -(R)-piperidin- 1 -carboxylic acid
tert- butyl ester:
By using the procedure described in Step-3 of Example- 1 above, and
by using trans-3-
[N’-(6-hydroxy-7-oxo-l,6-diaza-bicyclo[3.2.1]octane-2-carbonyl)-hydrazinocarbonyl]-(R)-
piperidin-1 -carboxylic acid tert-butyl ester (13.5 gm , 0.033 mol),
pyridine (70 ml) and pyridine sulfur trioxide complex (26.11 gm, 0.164
mol), 0.5 N aqueous potassium dihydrogen phosphate solution (400 ml) and
tetrabutylammonium sulphate (9.74 gm, 0.033 mol) to provide the title
compound in 25 gm quantity as a yellowish solid, in quantitative yield.
Analysis: MS (ES-)
as a salt = 490.0 (M-l) as a free sulfonic acid;
Step-4: trans-sulfuric acid
mono-[2-(N’-[(R)-piperidin-3-carbonyl]-hydrazinocarbonyl)-7-
oxo-l,6-diaza-bicyclo[3.2.1]oct-6-yl]ester:
By using the procedure described in Step-4 of Example- 1 above, and
by using tetrabutylammonium salt of
trans-3-[N’-(6-sulfooxy-7-oxo-l,6-diaza-bicyclo[3.2.1]octane-2-
carbonyl)-hydrazinocarbonyl]-(R)-piperidin-l-carboxylic acid tert-butyl
ester (24 gm , 0.032 mmol), dichloromethane (60 ml) and trifluoroacetic
acid (60 ml) to provide the title compound in 10 gm quantity as a white
solid, in 79% yield.
Analysis: MS (ES-)= C
13H
21N5O
7S = 390.2 (M-l) as a free sulfonic acid;
H
XNMR (DMSO-d
6) = 9.97 (d, 2H), 8.32 (br s,
2H), 4.00 (br s, IH), 3.81 (d, IH), 3.10-3.22 (m, 3H), 2.97-3.02 (m,
2H), 2.86-2.91 (m, IH), 2.65-2.66 (m, IH), 1.97-2.03 (m, IH), 1.57-1.88
(m, 7H).
-32.6°, (c 0.5, water).
PATENT
http://www.google.com/patents/WO2015059643A1?cl=en

Both, cefepime and a compound of Formula (I) may be present in the
composition in their free forms or in the form of their pharmaceutically
acceptable derivatives (such as salts, pro-drugs, metabolites, esters,
ethers, hydrates, polymorphs, solvates, complexes, or adducts).
Individual amounts of a compound of Formula (I) or a stereoisomer or a
pharmaceutically acceptable derivative thereof, and cefepime or
pharmaceutically acceptable derivative thereof in the composition may
vary depending on clinical requirements. In some embodiments, a compound
of Formula (I) or a stereoisomer or a pharmaceutically acceptable
derivative thereof in the composition is present in an amount from about
0.01 gram to about 10 gram. In some other embodiments, cefepime or a
pharmaceutically acceptable derivative thereof in the composition is
present in an amount from about 0.01 gram to about 10 gram.
PATENT
http://www.google.com/patents/WO2015063653A1?cl=en
PATENT
WO 2015110885
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015110885
Formula (I)

(a) hydrogenolysis of a compound of Formula (II) to obtain a compound of Formula (III);

convertin a compound of Formula (III) to a compound of Formula (IV);



Example 1
Synthesis of
(25, 5R)-7-oxo-6-sulphooxy-2-[((3R)-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane (I):
Step-1: Preparation of (25,
5R)-6-hydroxy-7-oxo-2-[((3R)-iV-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane
(III):
(25,
5i?)-6-benzyloxy-7-oxo-2-[((3i?)-N-Boc-piperidine-3-carbonyl)-hydrazino-carbonyl]
-l,6-diazabicyclo[3.2.1]octane (II) (130 g, 0.259 mol) was dissolved in
methanol (1040 ml) to obtain a clear solution. To this solution, was
added 10% palladium on carbon (13 g, 0.26 mol). The suspension was
stirred under 230-250 psi hydrogen atmosphere at temperature of about 30
°C for about 2 hour. The catalyst was filtered over celite bed and
catalyst containing bed was washed with additional methanol (400 ml).
The methanolic solution was re-filtered through fresh celite bed and
washed with methanol (100 ml). The filtrate was concentrated under
vacuum at temperature of about 30°C to obtain the off white solid as
product. The so obtained solid was stirred with cyclohexane (750 ml).
The solid was then filtered and washed with cyclohexane (320 ml) and
dried under suction to obtain 107 g of (25,
5i?)-6-hydroxy-7-oxo-2-[((3i?)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo
[3.2.1]octane (III).
Analysis:
Mass: 412.4 (M+l); for Molecular Formula of C18H29N5O6 and Molecular Weight of 411.5; and
Purity as determined by HPLC: 98.02%.
Step-2: Preparation of tetrabutylammonium salt of (25,
5R)-6-sulfooxy-7-oxo-2-[((3R)-iV-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,
6-diaza-bicyclo[3.2.1] octane (IV):
A solution of (25,
5i?)-6-hydroxy-7-oxo-2-[((3i?)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane
(III) (106 g, 0.26 mol) in dichloromethane was charged with triethyl
amine (110 ml, 0.78 mol) under stirring. To this clear solution was
added pyridine sulfur trioxide complex (82.5 g, 0.53 mol) under nitrogen
atmosphere and stirred at temperature of about 30°C for about 2 hour.
The reaction mixture was diluted with 0.5 N aqueous potassium dihydrogen
phosphate solution (2100 ml) followed by ethyl acetate (2100 ml). The
turbid solution was stirred for 15 minute and then the layers were
separated. The aqueous layer was washed with dichloromethane (530 ml)
and then with ethyl acetate (1060 ml). Tetrabutyl ammonium sulfate (79
g, 0.23 mol) was added to the separated aqueous layer and stirred for 12
hour. The extraction of the product was done using dichloromethane as
solvent (1150 ml x 2). The organic layer was dried over sodium sulfate
and then evaporated under vacuum at temperature below 40°C to furnish
108 g of tetrabutylammonium salt of (25,
5i?)-6-sulfooxy-7-oxo-2-[((3i?)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,
6-diaza-bicyclo
[3.2.1] octane (IV).
Analysis:
Mass: 490.3 (M-l) as free sulfonic acid; for Molecular Formula of Ci8H
28N50
9S.N(C4H9)4 and Molecular weight of 733.0; and
Purity as determined by HPLC: 86.50 %.
Step-3:
Preparation of (25, 5R)-7-oxo-6-sulphooxy-2-[((3R)-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane (I):
Tetrabutylammonium salt of (25,
5i?)-6-sulfooxy-7-oxo-2-[((3i?)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,
6-diaza-bicyclo[3.2.1]octane (IV) (88 g, 0.12 mol) was dissolved in
dichloromethane (225 ml). The reaction mass was cooled to about -10°C
and to this trifluoroacetic acid (225 ml) was added slowly. The reaction
mixture was stirred for 1 hour at temperature of about -10°C. The
solvent was removed under high vacuum at about 30°C. The residue (280 g)
was stirred with diethyl ether (1320 ml) for 1 hour. The precipitated
solid was filtered and the cake was washed with fresh diethyl ether (440
ml). This process was repeated with fresh diethyl ether (1320 ml + 440
ml). The obtained white solid was dried at temperature of about 30°C and
suspended in acetone (1320 ml). The pH of the suspension was adjusted
to 6.5-7.0 using 10% solution of sodium 2-ethyl hexanoate in acetone.
The resulting suspension was filtered under suction and the wet cake was
washed with acetone (440 ml) to provide the crude solid. The solid was
further dried under vacuum at 40°C to yield 40 g of (25,
5i?)-7-oxo-6-sulphooxy-2-[((3i?)-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane
(I).
Analysis:
Mass: 392.2 (M+l); for Molecular formula of C1
3H21N5O7S and Molecular Weight of 391.4;
Purity as determined by HPLC: 92.87%; and
Melting point as determined by DSC: 274°C.
Example 2
Synthesis of Pure (25,
5R)-7-oxo-6-sulphooxy-2-[((3R)-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane
(I):
Step-1: Preparation of (25,
5R)-6-hydroxy-7-oxo-2-[((3R)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane
(III):
The procedure for the synthesis of (25,
5i?)-6-hydroxy-7-oxo-2-[((3i?)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane
(III) is same as given in Step- 1 of Example 1.
Step-2: Preparation of tetrabutylammonium salt of (25,
5R)-6-sulfooxy-7-oxo-2-[((3R)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,
6-diaza-bicyclo[3.2.1] octane (IV):
A solution of (25,
5i?)-6-hydroxy-7-oxo-2-[((3i?)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane
(III) (106 g, 0.26 mol) in dichloromethane was charged with
triethylamine (110 ml, 0.78 mol) under stirring to provide a clear
solution. To this clear solution was added pyridine sulfur trioxide
complex (82.5 g, 0.53 mol) under nitrogen atmosphere and stirred at
temperature of about 30 °C for 2 hours. The reaction mixture was diluted
with 0.5 N aqueous potassium dihydrogen phosphate solution (2100 ml)
followed by ethyl acetate (2100 ml). The turbid solution was stirred for
15 minutes and then the layers were separated. The aqueous layer was
washed with dichloromethane (530 ml) and then with ethyl acetate (1060
ml) respectively. Tetrabutyl ammonium sulfate (79 g, 0.23 mol) was added
to the separated aqueous layer and stirred for 12 hours. The extraction
of the product was done using dichloromethane as solvent (1150 ml x 2).
Aliquot of the organic layer was dried over sodium sulfate for purity
check. Considering the purity of the product as obtained above, silica
gel (530 g) was added to the dichloromethane layer and stirred for 1
hour. This was filtered and again silica was taken in dichloromethane
(3200 ml) and stirred for 45 minutes and filtered. Combined
dichloromethane layer was filtered through the celite bed again and
washed with additional 200 ml dichloromethane. The solvent was removed
to obtain 88 g of tetrabutylammonium salt of (25,
5i?)-6-sulfooxy-7-oxo-2-[((3i?)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-!,
6-diaza-bicyclo[3.2.1]octane (IV) as white foam.
Analysis:
Mass: 490.3 (M-l) as a free sulfonic acid; for Molecular Formula of Ci8H
28N50
9S.N(C4H
9)4 and Molecular Weight of 733.0; and
Purity as determined by HPLC: 98.34%.
Step-3:
Preparation of (25, 5R)-7-oxo-6-sulphooxy-2-[((3R)-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane (I):
The above obtained tetrabutylammonium salt of (25,
5i?)-6-sulfooxy-7-oxo-2-[((3i?)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,
6-diaza-bicyclo[3.2.1]octane (IV) having purity of more than 98% (88 g,
0.12 mol) was dissolved in dichloromethane (225 ml). The reaction mass
was cooled to temperature of about -10°C and to this trifluoroacetic
acid (225 ml) was added slowly. The reaction mixture was stirred for 1
hour at about -10°C. The solvent was removed under high vacuum at
temperature of about 30°C. The residue (280 g) was stirred with diethyl
ether (1320 ml) for 1 hour. The precipitated solid was filtered and the
cake was washed with fresh diethyl ether (440 ml). This process was
repeated with fresh diethyl ether (1320 ml + 440 ml). The obtained white
solid was dried at about 30°C and suspended in acetone (1320 ml). The
pH of the suspension was adjusted to 6.5-7.0 using 10% solution of
sodium 2-ethyl hexanoate in acetone. The resulting suspension was
filtered under suction and the wet cake was washed with acetone (440 ml)
to provide the crude solid. The solid was further dried under vacuum at
40°C to yield 40 g of (25,
5i?)-7-oxo-6-sulphooxy-2-[((3i?)-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane
(I).
Analysis:
Mass: 392.2 (M+l); for Molecular Formula of C1
3H21N5O7S and Molecular Weight of 391.4; and
Purity as determined by HPLC: 98.7%.
Recovery of tetrabutylammonium salt of (25,
5R)-6-sulfooxy-7-oxo-2-[((3R)-iV-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]
octane (IV):
The silica recovered from the Step-2 was stirred with dichloromethane containing 2%
methanol (2000 ml) for one hour. Silica was filtered, washed with
additional same composition of solvents (500 ml). Combined
dichloromethane was filtered through the celite bed and washed with same
composition of solvents (200 ml), evaporated to afford 1 1 g of
tetrabutylammonium salt of (25,
5i?)-6-sulfooxy-7-oxo-2-[((3i?)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l
, 6-diaza-bicyclo[3.2.1] octane (IV) as off white solid.
Repeating Step-3 with the above obtained tetrabutylammonium salt of
(25, 5R)-6-sulfooxy-7-oxo-2-
[((3i?)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl] – 1 ,
6-diaza-bicyclo [3.2.1] octane (IV) produced additional 7 g of compound
of Formula (I).
Analysis:
Mass: 392.2 (M+l); for Molecular Formula of CnH^NsOvS and Molecular Weight of 391.4;
Purity as determined by HPLC: 98.7%; and
Assay as determined by HPLC: 104% against reference standard of compound of Formula (I).
Example 3
Preparation of
amorphous form of (25, 5R)-7-oxo-6-sulphooxy-2-[((3R)-piperidine-3-carbonyl)-hydrazinocarbonyl] – 1, 6-diaza-bicyclo[3.2. l]octane (I) :
Tetrabutylammonium salt of (25,
5i?)-6-sulfooxy-7-oxo-2-[((3i?)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,
6-diaza-bicyclo[3.2.1]octane (IV) (60 g, 0.081 mol), obtained in Step-2
of Example-2 was dissolved in dichloromethane (150 ml, 2.5 volume) to
obtain a clear solution. Reaction mass was cooled to about -10°C and to
it trifluoroacetic acid (150 ml) was slowly added. The reaction mixture
was stirred for 1 hour at about – 10°C. The solvent was removed under
high vacuum at about 30°C. Diethyl ether (600 ml x 3) was added to the
residue ( 184 g) and stirred for 15 minute every time. The solvent was
decanted off and the residue was washed with acetonitrile (600 ml x 3).
This process was also repeated with dichloromethane (600 ml x 3). The
off white solid was
isolated and dried under high vacuum at about 35 °C for 3 hour to
obtain 33 g of amorphous form of (25,
5i?)-7-oxo-6-sulphooxy-2-[((3i?)-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane
(I). The XRD is shown in Figure 1.
Analysis:
Mass: 392.2 (M+l); for Molecular Formula of C1
3H21N5O7S and Molecular Weight of 391.4;
HPLC purity: 92.26%; and
Melting point as determined by DSC: 210°C (loss of moisture below 100°C).
Example 4
Preparation of
crystalline form of (25, 5R)-7-oxo-6-sulpho-oxy-2-[((3R)-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane (I):
The (25,
5i?)-7-oxo-6-sulphooxy-2-[((3i?)-piperidine-3-carbonyl)-hydrazino
carbonyl]-l,6-diaza-bicyclo[3.2.1]octane (I) obtained as white solid (40
g) in Step-3 of Example 2 was dissolved in demineralised water (40 ml)
to obtain a clear solution. To this isopropyl alcohol (280 ml) was added
under stirring at room temperature. The obtained turbid solution became
sticky initially then slowly started to convert into white solid,
stirring continued for about 17 hours at temperature of about 30°C. The
precipitated solid was filtered and washed with water: isopropyl alcohol
mixture (20 ml: 140 ml). White solid was dried under high vacuum at
temperature of about 45 °C for 5 hours to get 34 g of crystalline form
of (25,
5i?)-7-oxo-6-sulphooxy-2-[((3i?)-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]
octane (I).
Analysis:
Mass: 392.2 (M+l) for Molecular Formula of C1
3H21N5O7S and Molecular Weight of 391.4;
Purity as determined by HPLC: 98.7%;
Assay as determined by HPLC: 104% against reference standard of compound of Formula (I); and
Melting point as determined by DSC: 278°C (9% loss of moisture at 143-152°C).
X-ray powder diffraction pattern comprising a peak selected from the
group consisting of 10.31 (± 0.2), 10.59 (± 0.2), 12.56 (± 0.2), 13.84
(± 0.2), 15.65 (± 0.2), 18.19 (± 0.2), 18.51(± 0.2), 20.38 (± 0.2),
20.65 (± 0.2), 24.30 (± 0.2), 24.85 (± 0.2) and 25.47 (± 0.2) degrees 2
theta.
PATENT
WO 2014135931
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014135931
Scheme 1.


Formula (I)
preparation of a compound of Formula (I), comprising:

Formula (I)
(a) reacting a compound of Formula (II) with a compound of Formula (III) to obtain a compound of Formula (IV);

Formula (II) Formula (III)

Formula (IV)
(b) hydrogenolysis of a compound of Formula (IV) to obtain a compound of Formula

X. Formula (V)
(c) sulfonating a compound of Formula (V) to obtain a compound of Formula (VI); and

Formula (VI)
(d) converting a compound of Formula (VI) into a compound of Formula (I).
Example -1
Preparation of (R)-N-Boc-piperidine-3-carboxylic acid hydrazide (II):
Step-1: Preparation of (R)-Ethyl-N-Boc-piperidine-3-carboxylate (VIII)
To a solution of (R)-N-Boc-piperidine-3-carboxylic acid (1 kg. 4.36
mol) in N,N-dimethylacetamide (3 L) was charged potassium carbonate
(0.664 kg, 4.80 mol) under mechanical stirring and the resulting
suspension was stirred for 30 minutes at room temperature. To the
reaction mass, ethyl iodide (0.75 kg, 4.80 mol) was charged via addition
funnel and the reaction mass was stirred for 15 minutes at room
temperature followed by at 50°C for 1 hour. The reaction was monitored
using TLC (ethyl acetate: hexane 1:1). After the reaction was complete,
the reaction mass was allowed to cool to room temperature and diluted
with ethyl acetate (5 L). The suspension was filtered under suction and
the wet cake was washed with ethyl acetate (5 L). The filtrate was
stirred with 5% w/v sodium thio sulfate (15 L) and layers were
separated. The aqueous layer was re-extracted with additional ethyl
acetate (5 L). The combined organic layer was washed with water (5 L)
and dried over sodium sulfate. The organic layer was evaporated under
vacuum to provide semi-solid which solidifies upon standing as
(R)-ethyl-N-Boc-piperidine-3-carboxylate in 1.1 kg quantity in 99.5%
yield.
Analysis:
NMR: (CDC13): 4.63 (q, 2H), 3.90 (d, 1H), 2.87-2.95 (m, 2H), 2.73
(td, 1H), 2.32-2.39 (m, 1H), 1.66-2.01 (m, 2H), 1.52-1.68 (m, 2H), 1.39
(s, 9H), 1.19 (t, 3H).
Mass: (M+l): 258.1 for C13H23N04;
Step-2: Preparation of (R)-N-Boc-piperidine-3-carboxylic acid hydrazide (II):
(R)-N-Boc-ethyl-piperidine-3-carboxylate (1.1 kg, 4.28 mol) was
liquefied by warming and transferred to a round bottom flask (10 L), to
this was charged hydrazine hydrate (0.470 kg, 9.41 mol) and stirring was
started. The reaction mixture was stirred at about 120°C to 125°C for 5
hours. As the TLC showed (Chloroform: methanol 9:1) completion of
reaction, the reaction mixture was cooled to room temperature and
diluted with water (5.5 L) followed by dichloromethane (11 L) and was
stirred for 20 minutes. The layers were separated and aqueous layer was
extracted with additional dichloro methane (5.5 L). Combined organic
layer was washed with water (2.75 L). The organic layer was dried over
sodium sulfate and evaporated under vacuum to provide a thick gel which
upon stirring and seeding in the presence of cyclohexane (5.5 L)
provided white solid. The suspension was filtered and wet cake was
washed with fresh cyclohexane (0.5 L). The cake was dried at 35°C under
vacuum to provide (R)-N-Boc-piperidine-3-carboxylic acid hydrazide as a
white solid in 0.90 kg quantity in 87% yield.
Analysis
NMR: (CDC13): 7.42 (br s, 1H), 3.92 (d, 1H), 3.88 (s, 2H), 3.54-3.65
(br s, 1H), 3.17 (br t, 1H), 2.98 (br s, 1H), 2.22-2.32 (br s, 1H),
1.82-1.90 (br m, 2H), 1.76 (s, 1H), 1.60-1.70 (m, 1H), 1.45 (s, 9H).
Mass (M+l): 244.1 for C11H21N303.
Specific rotation: [ ]
25D = -53.5° (c 0.5, Methanol).
HPLC purity: 99%
Example 2
Preparation of (2S,
5R)-7-oxo-6-sulphooxy-2-[((3R)-piperidine-3-carbonyl)-
hydrazinocarbonyl] -l,6-diaza-bicyclo[3.2.1]octane (I):
Step-1: Preparation of (2S, 5R)-
6-benzyloxy-7-oxo-2-[((3R)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]
– 1 ,6-diaza-bicyclo [3.2.1 ] octane(IV) :
Sodium (2S,
5R)-7-oxo-6-benzyloxy-l,6-diaza-bicyclo[3.2.1]octane-2-carboxylate (III,
200 gm, 0.67 mol; prepared using a method disclosed in Indian Patent
Application No 699/MUM/2013) was dissolved in water (2.8 L) to obtain a
clear solution under stirring at room temperature. To the clear solution
was added successively, (R)-N-Boc-piperidine-3-carboxylic acid
hydrazide (171 gm, 0.70 mol), EDC hydrochloride (193 gm, 1.01 mol), and
HOBt (90.6 gm, 0.67 mol) followed by water (0.56 L) under stirring at
35°C. The reaction mixture was stirred at 35°C for 20 hours. As maximum
precipitation was reached, TLC (acetone: hexane 35:65) showed completion
of reaction. The suspension was filtered under
suction and the wet cake was washed with additional water (2 L). The
wet cake was suspended in warm water (10 L) and stirred for 5 hours. It
was filtered under suction and dried under vacuum at 45°C to furnish
(2S,
5R)-6-benzyloxy-7-oxo-2-[((3R)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane
(IV) as a white powder in 270 gm quantity in 87% yield.
Analysis
NMR: (CDC13): 8.40 (br s, 1H), 7.34-7.44 (m, 5H), 5.05 (d, 1H), 4.90
(d, 1H), 4.00 (br d, 1H), 3.82 (br s, 1H), 3.30 (br s, 1H), 3.16-3.21
(m, 1H), 3.06 (br d, 1H), 2.42 (br s, 1H), 2.29-2.34 (m, 1H), 1.18-2.02
(m, 4H), 1.60-1.75 (m, 4H), 1.45-1.55 (m, 2H),1.44 (s, 9H).
Mass: (M+l) = 502.1 for C25H35N506
HPLC purity: 98.4%
Step-2: Preparation of (2S,
5R)-6-hydroxy-7-oxo-2-[((3R)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.
l]octane (V):
(2S,5R)-6-benzyloxy-7-oxo-2-[((3R)-N-Boc-piperidine-3-carbonyl)-hydrazino-carbonyl]-l,6-diaza-bicyclo[3.2.1]octane
(153 gm, 0.305 mol) was dissolved in methanol (1.23 L) to obtain a
clear solution. To this solution, was added 10% Pd-C (15.3 gm, 50% wet)
catalyst. The suspension was stirred for 3 hours under 100 psi hydrogen
atmosphere at 35°C. As reaction showed completion on TLC (TLC system
methanol: chloroform 10:90), the catalyst was filtered through celite
under suction. The catalyst was washed with additional methanol (600
ml). The filtrate was evaporated under vacuum below 40°C to provide a
crude residue. The residue was stirred with cyclohexane (1.23 L) for 1
hour. The solid was filtered at suction and the wet cake was washed with
additional cyclohexane (0.25 L) to furnish (2S,
5R)-6-hydroxy-7-oxo-2-[((3R)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2.1]octane
(V) in 125 gm quantity as a solid in quantitative yield. The product
being unstable was used immediately for the next reaction.
Analysis:
NMR: (CDC13): 9.0 (br s, 2H), 4.01 (br d, 2H), 3.80 (br s, 1H), 3.74
(br s, 1H), 3.48 (s, 1H), 3.13-3.26 (m, 3H), 2.96 (br s, 1H), 2.47 (br
s, 1H), 2.28-2.32 ( br dd, 1H), 2.08 (br s, 1H), 1.90-2.0 (m,
3H),1.65-1.80 (m, 3H) 1.44 (s, 9H).
Mass: (M-l): 410.3 for C18H29N506
HPLC purity: 96.34%
Step-3: Preparation of Tetrabutyl ammonium salt of (2S,
5R)-6-sulfooxy-7-oxo-2-[((3R)-N-Boc-piperidine-3-carbonyl)-hydrazinocarbonyl]-
1 ,6-diaza-bicyclo[3.2.1 ] octane (VI) :
A solution of (2S,
5R)-6-hydroxy-7-oxo-2-[((3R)-N-Boc-piperidine-3-carbonyl)-hydrazino
carbonyl]-l,6-diaza-bicyclo[3.2.1]octane (113 gm, 0.274 mol), in
dichloromethane (1.13 L) was charged with triethylamine (77 ml, 0.548
mol) under stirring to provide a clear solution. To the clear solution,
was added pyridine sulfur trioxide complex (57 gm, 0.356 mol) under
stirring at 35°C. The reaction mixture was stirred for 3 hours. The
reaction mixture was worked up by adding 0.5 M aqueous potassium
dihydrogen phosphate (1.13 L) followed by ethyl acetate (2.26 L) and the
biphasic mixture was stirred for 15 minutes at 35°C. Layers were
separated. Aqueous layer was re-extracted with dichloromethane ethyl
acetate mixture (1:2 v/v, 2.26 L twice). Layers were separated. To the
aqueous layer, was added solid tetrabutyl ammonium hydrogen sulfate (84
gm, 0.247 mol) and stirring was continued for 3 hours at room
temperature. Dichloromethane (1.13 L) was added to the reaction mixture.
Layers were separated. The aqueous layer was re-extracted with
additional dichloromethane (0.565 L). Layers were separated. To the
combined organic layer was added silica gel (226 gm) and the suspension
was stirred for 1 hour. Suspension was filtered and silica gel was
washed with dichloromethane (1 L). The combined filtrate was evaporated
under vacuum to provide solid mass. To the solid mass was added
cyclohexane (0.9 L) and stirred till complete solidification occurred
(about 1 to 2 hours). The suspension was filtered under suction and the
wet cake was dried under vacuum below 40°C to furnish tetrabutyl
ammonium salt of (2S,
5R)-6-sulfooxy-7-oxo-2-[((3R)-N-Boc-piperidine-3-carbonyl)-hydrazino
carbonyl]-l,6-diaza-bicyclo[3.2.1]octane (VI) as a white solid in 122 gm
quantity in 60% yield.
Analysis
NMR: (CDC13): 8.50 (br s, 2H), 4.32 (br s, 1H), 3.97 (d, 2H),
3.15-3.37 (m, 12H), 2.43 (br s, 1H), 2.33 (d, 1H), 2.10-2.2 (br m, 1H),
1.84-1.95 (m, 3H), 1.60-1.73 (m, 13H), 1.39-1.48 (m, 19H), 0.98 (t,
12H).
Mass: (M-l): 490.4 as a free sulfonic acid for C18H28N509S.N(C4H9)4;
HPLC purity: 96.3%
Step-4:
Synthesis of (2S, 5R)-6-sulfooxy-7-oxo-2-[((3R)-piperidine-3-carbonyl)-hydrazinocarbonyl]-l,6-diaza-bicyclo[3.2. l]octane (I):
Tetra-butyl ammonium salt of (2S,
5R)-6-sulfooxy-7-oxo-2-[((3R)-N-Boc-piperidine-3-carbonyl)-hydrazino
carbonyl]-l,6-diaza-bicyclo[3.2.1]octane (113 gm, 0.154 mol) was
dissolved in dichloromethane (280 ml) and to the clear solution was
slowly added trifluoroacetic acid (280 ml) between 0 to 5°C. The
reaction mixture was stirred between 0 to 5°C for 1 hour. The solvent
and excess trifluoroacetic acid was evaporated under vacuum below 40°C
to approximately 1/3 of it’s original volume to provide pale yellow oily
residue. The oily residue was stirred with diethyl ether (2.25 L) for 1
hour to provide a suspension. The precipitate was filtered under
suction and transferred to a round bottom flask, to it was added diethyl
ether (1.1 L) under stirring. The suspension was stirred for 30 minutes
and filtered under suction to provide a solid. The solid was charged in
a round bottom flask and to it was added acetone (1.130 L). The pH of
suspension was adjusted to 4.5 to 5.5 by adding 10% solution of
sodium-2-ethyl hexanoate in acetone carefully. The resulting suspension
was filtered under suction and the wet cake was washed with acetone (550
ml) to provide a crude solid. The obtained solid was dried under vacuum
below 40°C to furnish 65 gm of a crude mass. The crude mass was
dissolved in water (65 ml) under stirring and to the clear solution was
added isopropyl alcohol (455 ml). The suspension was stirred for 24
hours and filtered under suction. The wet cake was washed with isopropyl
alcohol (225 ml) and dried under vacuum below 40°C to provide a
crystalline
(2S, 5R)-6-sulfooxy-7-oxo-2-[((3R)-piperidine-3-carbonyl)-hydrazino carbonyl]-l,6-diaza-bicyclo[3.2.1]octane (I) free from impurities in 48 gm quantity in 80% yield.
Analysis:
NMR: (DMSO-d6) = 9.97 (d, 2H), 8.32 (br
s, 2H), 4.00 (br s, IH), 3.81 (d, IH), 3.10-3.22 (m, 3H), 2.97-3.02 (m,
2H), 2.86-2.91 (m, IH), 2.65-2.66 (m, IH), 1.97-2.03 (m, IH), 1.57-1.88
(m, 7H).
Mass: (M-l): 390.3 for C13H21N507S
HPLC purity: 95.78%
Specific rotation: [(X]25D: – 32.6° (c 0.5, water)
X-ray powder diffraction pattern comprising peak at (2 Theta Values):
10.28 (+ 0.2), 10.57 (± 0.2), 12.53 (± 0.2), 13.82 (± 0.2), 15.62 (±
0.2), 18.16 (± 0.2), 18.49 (± 0.2), 20.35 (+ 0.2), 20.64 (± 0.2), 21.33
(+ 0.2), 22.99 (+ 0.2), 23.18 (+ 0.2), 24.27 (± 0.2), 24.81 (+ 0.2),
25.45 (± 0.2), 29.85 (+ 0.2), 30.45 (± 0.2), 32.39 (+ 0.2), 36.84 (±
0.2).
REFERENCES
Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of
WCK-5107 Alone and in Combination With Cefepime (NCT02532140)
https://clinicaltrials.gov/show/NCT02532140
ClinicalTrials.gov Web Site 2015, September 01, To evaluate the
safety,tolerability and pharmacokinetics of single intravenous doses of
WCK 5107 alone and in combination with cefepime in healthy adult human
subjects.
| WO2013030733A1 * |
Aug 24, 2012 |
Mar 7, 2013 |
Wockhardt Limited |
1,6- diazabicyclo [3,2,1] octan-7-one derivatives and their use in the treatment of bacterial infections |
| WO2014135931A1 * |
Oct 12, 2013 |
Sep 12, 2014 |
Wockhardt Limited |
A process for preparation of (2s,
5r)-7-oxo-6-sulphooxy-2-[((3r)-piperidine-3-carbonyl)-hydrazino
carbonyl]-1,6-diaza-bicyclo [3.2.1]- octane |
| IB2012054290W |
|
|
|
Title not available |


 Mr Habil Khorakiwala, Chairman, Wockhardt Ltd.
Mr Habil Khorakiwala, Chairman, Wockhardt Ltd.


C1C[C@H](CNC1)C(=O)NNC(=O)[C@@H]2CC[C@@H]3C[N@]2C(=O)N3OS(=O)(=O)O
or
O=C(NNC(=O)[C@@H]2CC[C@@H]1CN2C(=O)N1OS(=O)(=O)O)[C@@H]3CCCNC3
C1CC(CNC1)C(=O)NNC(=O)C2CCC3CN2C(=O)N3OS(=O)(=O)[O-].[Na+]
Share this:
//////////
see..........
http://newdrugapprovals.org/2015/11/23/wck-5107-in-phase-1-from-wockhardt/































