Tesmilifene
BMS-217380; BMY-33419; DPPE
CAS No. 98774-23-3(Tesmilifene), 92981-78-7(Tesmilifene hydrochloride)
Tesmilifene
CAS 98774-23-3
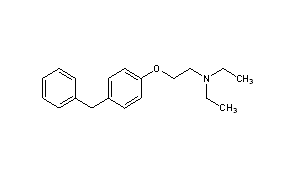
N,N-Diethyl-2-[4-(phenylmethyl)phenoxy]ethanamine
DPPE
MFC19H25NO
MW 283.41
Percent Composition: C 80.52%, H 8.89%, N 4.94%, O 5.65%

Hydrochloride
CAS 92981-78-7
BMS-217380-01; BMY-33419
MF C19H25NO.HCl
MF 319.87
Percent Composition: C 71.34%, H 8.19%, N 4.38%, O 5.00%, Cl 11.08%
Properties: White crystals from isopropanol + acetone (3:1), mp 156-158°. pKa 10.9.
Melting point: mp 156-158°
pKa: pKa 10.9
Therap-Cat: Antineoplastic adjunct (chemosensitizer).

AT YM BIOSCIENCES, GILEAD
Tesmilifene is a novel potentiator of chemotherapy which, when added to
doxorubicin, achieved an unexpected and very large survival advantage over
doxorubicin alone in a randomized trial in advanced breast cancer.
PHASE 23 FOR An estrogen receptor antagonist potentially for the treatment of advanced breast cancer, gastric cancer
Tesmilifene is a novel agent that augments cytotoxicity of various chemotherapeutic agents both
in vitro and
in vivo.
It binds selectively to the high-affinity microsomal antiestrogen
binding site (Ki=50nm) but has no affinity for estrogen receptors.
Inhibits concanavalin-A-induced histamine release in mast cells and acts
as a novel antagonist of intracellular histamine.
US 4803227


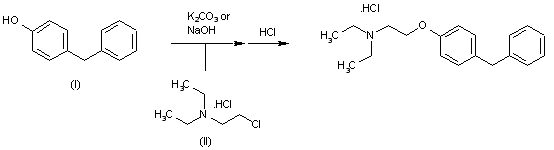
The
target product can be prepared by reacting para-benzylphenol (I) with
2-diethylaminoethylchloride hydrochloride (II) either by means of NaOH
in H2O or with K2CO3 in DMF/acetone (at 60 C in both cases), followed by
treatment with HCl to obtain the corresponding hydrochloride salt.
| | EP 0153160; JP 1985190742; US 4803227 |
US 4803227
http://www.google.com/patents/US4803227
Tesmilifene
is a small molecule chemopotentiator under development by YM
BioSciences, a Candian pharmaceutical company that specialises in the
development of cancer treatments. It is indicated for use in combination
with standard cytotoxic drugs, such as taxanes and anthracyclines,
which are widely used in the treatment of metastatic disease – when
cancers spread to distant sites in the body.
Tesmilifene, the
company's lead investigational compound, is currently in phase III
development for patients with metastatic breast cancer. At the end of
January 2007, an independent safety monitoring board advised the company
that its ongoing registration trial should be stopped; it was
considered unlikely that significant differences in overall survival
(primary endpoint) between treatment arms would emerge over time. The
company had hoped that the addition of tesmilifene to standard
epirubicin/cyclophosphamide therapy would confer a survival benefit
similar to that seen in its earlier phase III trial.
In light of
these disappointing results, YM BioSciences plans a detailed analysis of
its phase III data in advanced breast cancer to see if it can identify
why tesmilifene failed to add clinical benefit in this trial.
DRUG RESISTANCE LIMITS EFFECTIVENESS OF CHEMOTHERAPY
Cytotoxic drugs have proved potent weapons in the fight against malignant tumours and are considered first-line therapy for
the treatment of many cancers. However, while patients often respond
well to a first course of chemotherapy over time the response to drug
treatment diminishes and the tumour may eventually become drug
resistant. In some cases resistance can develop across several classes
of anti-cancer drugs, leading to multidrug resistance. The development
of drug resistance limits the effectiveness of many anti-cancer agents
and is an important contributor to cancer deaths.
The development
of agents that can overcome drug resistance is seen as one of the most
important areas of cancer research and for which there is significant
unmet need. Various approaches are being explored to boost the use of
cytotoxic agents including chemopotentiators, chemoprotectants and
liposomal formulations.
Clearly any agent that can prevent or
reverse drug resistance would have a major impact on treatment
strategies, enhancing the benefits of standard cytotoxic drugs.
TESMILIFENE MAY BOOST CYTOTOXIC EFFECTS OF ANTHRACYCLINES
Anthracyclines
are a class of cytotoxic agents with proven efficacy in the treatment
of breast cancer. They include agents such as doxorubicin and epirubicin
among others. Because patients with metastatic breast cancer may have
received anthracycline therapy for earlier stage breast cancer (adjuvant
therapy) or following disease recurrence, there is a risk that they
will fail to respond to continued treatment.
A phase III trial in
305 patients with advanced breast cancer has shown that when tesmilifene
is combined with doxorubicin it appears to improve survival over
treatment with doxorubicin alone. In this trial approximately half the
patients were treated with both tesmilifene and doxorubicin, while the
other half received doxorubicin alone. Although there were no
significant differences in tumour response rates, progression-free
survival, or average duration of response between treatment arms at
endpoint, overall survival was significantly improved in the combination
arm. Among patients treated with tesmilifene and doxorubicin overall
survival was 23.6 months compared with 15.6 months for those treated
with doxorubicin alone.
Researchers have suggested that tesmilifene may enhance the anti-tumour effects of anthracyclines in several ways:
- Reducing the cancer cell's ability to become resistant
- Decreasing the metabolism or "break-down" of doxorubicin
- Disrupting the cancer cell's energy source
TESMILIFENE REGISTRATION TRIAL
In
March 2004 YM BioSciences began its pivotal international phase III
trial of tesmilifene in metastatic breast cancer. By September 2005, 723
patients had been enrolled in the trial, which was designed once again
to compare the efficacy and safety of tesmilifene and an antrhacycline
(epirubicin) with epirubicin alone.
"At
the end of January 2007, an independent safety monitoring board advised
the company that its ongoing registration trial should be stopped."
Given
the survival benefit seen in the earlier trial, which was carried out
by the Canadian National Cancer Institute, the company was optimistic
about outcome in its pivotal registration trial. However, an interim
analysis of 351 events suggested that significant differences in overall
survival were unlikely to be seen between the two treatment arms as the
data matured and the trial was brought to a premature end.
In
addition to its work on anthracyclines, YM BioSciences has also been
exploring the potential of tesmilifene to enhance the efficacy of
taxanes, also standard chemotherapy for metastatic breast cancer. Other
potential applications include:
- Adjuvant therapy for breast cancer, i.e. immediately post-surgery and before the cancer has recurred or metastasised
- Hormone-refractory prostate cancer
- Lung cancer
- Non-Hodgkin's lymphoma
MARKETING COMMENTARY
Although
there have been major advances in the treatment of breast cancer in the
last 10 to 15 years, it remains a disease for which improved treatments
are still urgently needed. Estimates from the WHO suggest that
metastatic breast cancer will claim the lives of over 40,000 patients a
year.
Current treatments for metastatic breast cancer are rarely
curative but can nonetheless do much to improve patients' quality of
life or duration of survival. . By boosting the cytotoxic effects of
standard chemotherapy agents such as anthracyclines, while protecting
healthy cells, tesmilifene was thought to have potential to extend the
benefits of cytotoxic therapy to more patients. This is now in doubt
following premature ending of its pivotal registration trial in advanced
breast cancer.
Intracellular histamine antagonist with chemopotentiating and cytoprotective activity. Structurally similar to tamoxifen,
q.v., although binds anti-estrogen binding site (AEBS) with no affinity for the estrogen receptor.
Prepn: L. J. Brandes, M. W. Hermonat,
Biochem. Biophys. Res. Commun. 123, 724 (1984); and use as antineoplastic:
eidem,
US 4803227 (1989 to Univ. Manitoba); and study of binding affinity: M. Poirot
et al.,
Bioorg. Med. Chem. 8, 2007 (2000). Spectral analysis of interaction with P450 isozymes: L. J. Brandes
et al.,
Cancer Chemother. Pharmacol. 45, 298 (2000).
Clinical evaluation in combination with cyclophosphamide in prostate cancer: L. J. Brandes
et al.,
J. Clin. Oncol. 13, 1398 (1995); in combination with doxorubicin in breast cancer: L. Reyno
et al.,
J. Clin. Oncol. 22, 269 (2004).
Bioorg Med Chem 2000,8(8),2007
Product Literature References
Enhancement
of cytotoxicity of natural product drugs against multidrug resistant
variant cell lines of human head and neck squamous cell carcinoma and
breast carcinoma by tesmilifene.: P. J. Ferguson, et al.; Cancer Lett.
274, 279 (2009),
Abstract;
Phase
III study of N,N-diethyl-2-[4-(phenylmethyl) phenoxy]ethanamine
(BMS-217380-01) combined with doxorubicin versus doxorubicin alone in
metastatic/recurrent breast cancer: National Cancer Institute of Canada
Clinical Trials Group St: L. Reyno, et al.; J. Clin. Oncol.
22, 269 (2004),
Abstract;
Synergy between tamoxifen and cisplatin in human melanoma cells is dependent on the presence of antiestrogen-binding sites.: J.A. Jones, et al.; Cancer Res.
57, 2657 (1997),
Abstract;
Influence of DPPE on histamine release from isolated rat mast cells.: N. Grosman; Agents Actions
41, 1 (1994),
Abstract;
Histamine is an intracellular messenger mediating platelet aggregation.: S.P: Saxena, et al.; Science
243, 1596 (1989),
Abstract;
///////Tesmilifene, Antineoplastic Adjunct, Chemosensitizer, PHASE 3,
Tesmilifene hydrochloride, BMY-33419, BMS-217380, DPPE, N,N-DPPE, Antagonist of intracellular histamine
CCN(CC)CCOC1=CC=C(C=C1)CC2=CC=CC=C2
see............
http://newdrugapprovals.org/2015/12/04/tesmilifene/

 .
.




 .
.