ALISKIREN
(2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethylethyl)-4-hydroxy-7-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-8-methyl-2-(propan-2-yl)nonanamide, CAS 173334-57-1, base
CAS 173334-58-2,aliskiren hemifumarate
Aliskiren is a renin inhibitor. It was approved by the U.S. Food and Drug Administration in 2007 for the treatment of hypertension.
2-C30-H53-N3-O6.C4-H4-O4
1219.599
Novartis (Originator), Speedel (Licensee)
CARDIOVASCULAR DRUGS, Heart Failure Therapy, Hypertension, Treatment of, Renal Failure, Agents for, RENAL-UROLOGIC DRUGS, Treatment of Renal Diseases, Renin Inhibitors
Tekturna contains aliskiren hemifumarate, a renin inhibitor, that is provided as tablets for oral administration. Aliskiren hemifumarate is chemically described as (2S,4S,5S,7S)-N-(2-carbamoyl-2-methylpropyl)-5-amino-4-hydroxy-2,7diisopropyl-8-[4-methoxy-3-(3-methoxypropoxy)phenyl]-octanamide hemifumarate and its structural formula is
Molecular formula: C30H53N3O6 • 0.5 C4H4O4
Aliskiren hemifumarate is a white to slightly yellowish crystalline powder with a molecular weight of 609.8 (free base- 551.8). It is soluble in phosphate buffer, n-octanol, and highly soluble in water.
|
Aliskiren (INN) (trade names Tekturna, US; Rasilez, UK and elsewhere) is the first in a class of drugs called direct renin inhibitors. Its current licensed indication is essential (primary) hypertension.
Aliskiren was co-developed by the Swiss pharmaceutical companies Novartis andSpeedel.[1][2] It was approved by the US Food and Drug Administration in 2007 for the treatment of primary hypertension.[3]
In December 2011, Novartis had to halt a clinical trial of the drug after discovering increased incidence of nonfatal stroke, renal complications, hyperkalemia, and hypotension in patients with diabetes and renal impairment (ALTITUDE Trial ).[4] [5]
As a result, in April 20, 2012:
A new contraindication was added to the product label concerning the use of aliskiren with angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors (ACEIs) in patients with diabetes because of the risk of renal impairment, hypotension, and hyperkalemia.
A warning to avoid use of aliskiren with ARBs or ACEIs was also added for patients with moderate to severe renal impairment (i.e., where glomerular filtration rate is less than 60 ml/min).
Renin, the first enzyme in the renin-angiotensin-aldosterone system, plays a role in blood pressure control. It cleaves angiotensinogen to angiotensin I, which is in turn converted byangiotensin-converting enzyme (ACE) to angiotensin II. Angiotensin II has both direct and indirect effects on blood pressure. It directly causes arterial smooth muscle to contract, leading to vasoconstriction and increased blood pressure. Angiotensin II also stimulates the production of aldosterone from the adrenal cortex, which causes the tubules of the kidneys to increase reabsorption of sodium, with water following, thereby increasing plasma volume, and thus blood pressure. Aliskiren binds to the S3bp binding site of renin, essential for its activity.[6] Binding to this pocket prevents the conversion of angiotensinogen to angiotensin I. Aliskiren is also available as combination therapy withhydrochlorothiazide.[7]
Many drugs control blood pressure by interfering with angiotensin or aldosterone. However, when these drugs are used chronically, the body increases renin production, which drives blood pressure up again. Therefore, doctors have been looking for a drug to inhibit renin directly. Aliskiren is the first drug to do so.[8][9]
Aliskiren may have renoprotective effects independent of its blood pressure−lowering effect in patients with hypertension, type 2 diabetes, and nephropathy, who are receiving the recommended renoprotective treatment. According to the AVOID study, researchers found that treatment with 300 mg of aliskiren daily, as compared with placebo, reduced the mean urinary albumin-to-creatinine ratio by 20%, with a reduction of 50% or more in 24.7% of the patients who received aliskiren as compared with 12.5% of those who received placebo. Furthermore, the AVOID trial showed treatment with 300 mg of aliskiren daily reduces albuminuria in patients with hypertension, type 2 diabetes, and proteinuria, who are receiving the recommended maximal renoprotective treatment with losartan and optimal antihypertensive therapy. Therefore, direct renin inhibition will have a critical role in strategic renoprotective pharmacotherapy, in conjunction with dual blockade of the renin−angiotensin−aldosterone system with the use of ACE inhibitors and angiotensin II–receptor blockers, very high doses of angiotensin II−receptor blockers, and aldosterone blockade.[10]
Aliskiren is a minor substrate of CYP3A4 and, more important, P-glycoprotein:
- It reduces furosemide blood concentration.
- Atorvastatin may increase blood concentration, but no dose adjustment is needed.
- Due to possible interaction with ciclosporin, the concomitant use of ciclosporin and aliskiren is contraindicated.
- Caution should be exercised when aliskiren is administered with ketoconazole or other moderate P-gp inhibitors (itraconazole, clarithromycin, telithromycin, erythromycin, or amiodarone).
- Doctors should stop prescribing aliskiren-containing medicines to patients with diabetes (type 1 or type 2) or with moderate to severe kidney impairment who are also taking an ACE inhibitor or ARB, and should consider alternative antihypertensive treatment as necessary.[13]
- Aliskiren (I) is a second generation renin inhibitor with renin-angiotensin system (RAS) as its target. It’s used clinically in the form of Aliskiren hemifumarate (Rasilez®) and was approved by FDA in May, 2007.
- Aliskiren has the chemical name: (2S, 4S, 5S, 7S)-5-amino-N-(2-carbamoyl-2-methylpropyl)-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methyloctanamide (CAS No.: 173334-57-1). Its chemical structure is illustrated with Formula I given below:
- The method of preparation for Aliskiren and its intermediates has been reported in US7132569 , WO0208172 , US5559111 (equivalent patent toCN1266118 ), US5606078 , CN101016253 , WO2007/045421 ,EP2062874 , Helvetica ChimicaActa (2005, 3263-3273).
- In US7132569 , WO0208172 et al., the preparation of Aliskiren (I) comprises the following steps as described in reaction scheme 1: coupling 2-(3-methoxypropoxy)-4-((R)-2-(bromomethyl)-3-methylbutyl)-1-methoxybenzene (II) with (2S, 4E)-5-chloro-2-isopropyl-4-pentenoic acid derivative (III) to obtain the compound of formula IV; halolactonization of the compound of formula IV to obtain the compound of formula V; then substituting the compound of formula V with azide to obtain the compound of formula VI; ring-opening the compound of formula VI with 3-amino-2,2-dimethylpropionamide (VII) in the presence of 2-hydroxypyridine and triethylamine to obtain the compound of formula VIII and a final catalytic hydrogenation of the compound of formula VIII to obtain Aliskiren (I). This preparation process is illustrated in Reaction Scheme 1.
- In the patented preparation described above, chiral starting materials with the compounds of formula II and III are utilized to obtain the compound of formula IV. However, the reactions followed after the preparation of the compound of formula IV, such as the halolactonization and especially the substitutive reaction between the compound of formula V and azide, have problems of low yields and numerous by-products, which is not conducive to industrial scale production.
- In the patented preparation described above, there are multiple reaction steps in the preparation of the compound of formula X from the compound of formula XII. The key steps, as described in Reaction Scheme 3, involve selective reduction agents such as sodium tri-tert-butoxyaluminum hydride and diisobutylaluminium hydride to prepare aldehyde and the reaction conditions need to be very well-controlled.
- [0009]The compound of formula XI prepared by reaction scheme 2 could then be converted into Aliskiren (I) after multiple catalytic hydrogenation, protection and de-protection. In this method of preparation, a stepwise catalytic hydrogenation, azido reduction and dehydroxylation were implemented to reduce by-products during the catalytic hydrogenation. In addition, it is necessary to protect and de-protect the free hydroxyl group during the preparation. This synthetic scheme has disadvantage of multiple synthetic steps, tedious operation, lengthy overall reaction duration, low yield and particularly high production cost for the starting compound of formula X.
- WO2007/045421 has reported an improved preparation method in which the starting material 4-bromo-1-methoxy-2-(3-methoxypropoxy)benzene (IX) firstly reacts with the compound of formula XIII via Grignard reaction to obtain the compound of formula XIV, and then followed by catalytic hydrogenation and ketone reduction to yield the compound of formula XV-A, as illustrated in Reaction Scheme 4:
- In the above preparation, expensive reagents, such as sodium tri-tert-butoxyaluminum hydride and diisobutylaluminium hydride were eliminated, but additional synthetic steps were introduced. In addition, the preparation of the compound of formula XV-A prepared from the compound of formula XIV via ketone reduction required extended reaction time, great amount of catalyst with multiple small addition and good operation skills.
- EP2062874A1 provides a method in preparing the compound of formula XVI. In this method, the compound of formula XVII is obtained from the compound of formula XVI via halogenation. A corresponding Grignard reagent is firstly prepared from the compound of formula IX or XVII reacting with magnesium, which is then couples with another chemical in the presence of the metal catalyst iron(III) acetylacetonate (Fe(acac)3) to obtain the compound of formula XVIII as described in Reaction Scheme 5:
- In EP2062874A1 , the compound of formula XVIII reacts with 3-amino-2,2-dimethylpropionamide (VII). The resulted product is then through reduction of the azio group to obtain Aliskiren (I). In this patent, detailed experimental protocol was not provided although N-methylpyrrolidone was mentioned as solvent. We found: 1) it is difficult to prepare the Grgnard reagent from the compound of formula IX; 2) the compounds of formula XVII and XVIII are not quite stable in the presence of iron(III) acetylacetonate. In addition, the yield in preparing the compound of formula XVIII was extremely low.
the spiro aldehyde (XLVII) is treated with N-benzylhydroxylamine in dichloromethane to give nitrone (LII), which is submitted to a Grignard reaction with the magnesium derivative of intermediate (XXX) in THF to afford the adduct (LIII) as a mixture of epimers at the amino group. Simultaneous N-dehydroxylation and cleavage of the spiro function of (LIII) by means of Zn, Cu (OAc) 2 in AcOH / water gives lactone (LIV), which is condensed with 3-amino- 2,2-dimethylpropionamide (XIX) by means of TEA and 2-hydroxypyridine giving the adduct (LV). Finally, the benzylamino group of (LV) is removed with H2 over Pd / C in methanol to yield a mixture of two epimers at the amino group, from which aliskiren is separated.
Tetrahedron Lett2001, 42, (29): 4819
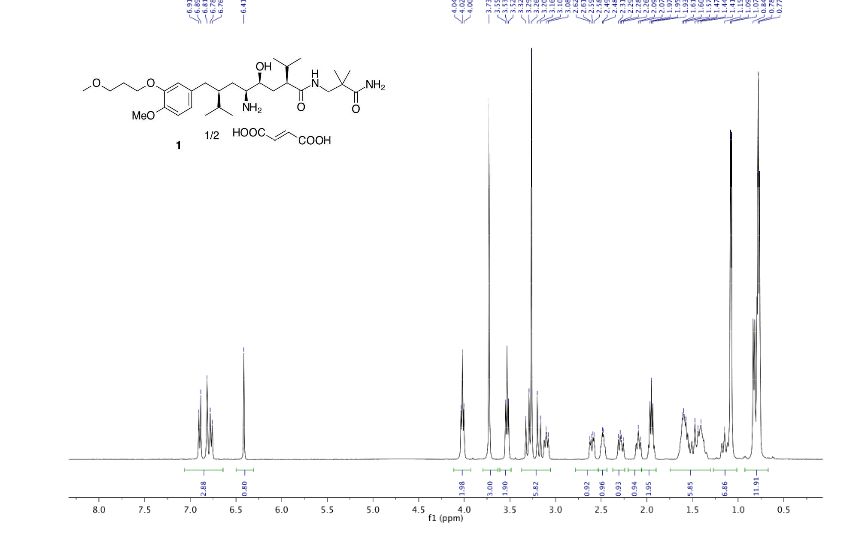
NMR
ALISKIREN BASE
MS m/z: 552.6 (M+H)+; 1H-NMR (400 MHz, CDCl3) δ 6.88-6.75 (m, 3H), 4.08-4.04 (t, J = 6.3Hz, 2H), 3.79 (s, 3H), 3.60-3.55 (t, J = 6.3Hz, 2H), 3.30 (s, 3H), 3.30-3.25 (m, 3H), 2.69 (m, 2H), 2.49 (m, 1H), 2.27 (m, 1H), 2.04 (m, 2H), 1.78-1.35 (m, 7H), 1.10 (m, 6H), 0.90 (m, 12H) ppm.


Paper
A novel synthesis of the renin inhibitor aliskiren based on an unprecedented disconnection between C5 and C6 was developed, in which the C5 carbon acts as a nucleophile and the amino group is introduced by a Curtius rearrangement, which follows a simultaneous stereocontrolled generation of the C4 and C5 stereogenic centers by an asymmetric hydrogenation. Operational simplicity, step economy, and a good overall yield makes this synthesis amenable to manufacture on scale.
Convergent Synthesis of the Renin Inhibitor Aliskiren Based on C5–C6 Disconnection and CO2H–NH2 Equivalence
† Dipartimento di Biotecnologie, Chimica e Farmacia, Università degli Studi di Siena, Via A. Moro 2, 53100 Siena, Italy
‡Chemessentia SRL, Via Bovio 6, 28100 Novara, Italy
§ Dipartimento di Chimica e Chimica Industriale, Università degli Studi di Genova, Via Dodecaneso 31, 16146 Genova, Italy
∥ Dipartimento di Chimica, Materiali e Ingegneria Chimica “G. Natta”, Politecnico di Milano, Via Mancinelli 7, 20131 Milano, Italy
⊥Johnson Matthey Catalysis and Chiral Technologies, 28 Cambridge Science Park, Milton Road, Cambridge CB4 0FP, United Kingdom
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.5b00396
Publication Date (Web): January 5, 2016
Copyright © 2016 American Chemical Society
*E-mail: mraspari@ITS.JNJ.com., *E-mail: maurizio.taddei@unisi.it.
PAPER
PAPER
EP 0678500; EP 0678503; JP 1996053434; JP 1996081430; US 5559111; US 5627182; US 5646143
Alkylation of 3-hydroxy-4-methoxybenzyl alcohol (I) with 1-bromo-3-methoxypropane (II) gives ether (III). Subsequent conversion of benzyl alcohol (III) into bromide (IV) is carried out using bromotrimetylsilane. The chiral isovaleryloxazolidinone (V) is alkylated with bromide (IV) by means of LiHMDS to afford (VI), which is hydrolyzed to the (S)-2-aryl-2-isopropylpropionic acid (VII) by means of lithium peroxide. The reduction of acid (VII) to the corresponding alcohol with NaBH4/I2 reagent, followed by treatment with PPh3 and NBS, provides bromide (VIII). Alkylation of the chiral dimethoxydihydropyrazin (IX) with bromide (VIII) produces (X). Further hydrolysis of the pyrazine ring of (X) with HCl, followed by Boc protection of the resulting (S,S)-amino ester, yields compound (XI). Reduction of the ester group of (XI) with DIBAL gives aldehyde (XII). This compound is condensed with the Grignard reagent (XIII) to afford the diastereomeric mixture of amino alcohols (XIV). Treatment of mixture (XIV) with 2,2-dimethoxypropane (XV) and TsOH produces a mixture of oxazolidines, from which the required (S,S,S)-isomer (XVI) is isolated by flash chromatography. Hydrogenolitic deprotection of the benzyl ether of (XVI) gives alcohol (XVII).
This alcohol is oxidized to aldehyde with NMMO and tetrapropylammonium perruthenate (TPAP), and further oxidized to carboxylic acid (XVIII) with KMnO4 and tetrabutylammonium bromide (TBAB). Coupling of (XVIII) with aminoamide (XIX) by means of diethyl cyanophosphonate and TEA gives (XX). Finally, acid hydrolysis of the oxazolidine ring and Boc protecting groups of (XX) furnishes the corresponding amino alcohol, which is finally converted to the hemifumarate salt.
WO 0109079; WO 0109083
Alternatively, the chiral azido intermediate (XXXIV) can also be synthesized as follows: Alkylation of oxazolidinone (V) with 1-chloro-3-iodopropene (XLVIII) by means of LiHMDS in THF gives compound (XLIX), which is condensed with the magnesium derivative of the phenylpropyl chloride (XXX) to yield, after working up, amide (L). Bromination of (L) with NBS and phosphoric acid affords the bromolactone (LI), which by treatment with NaN3 in tripropylene glycol/water provides the azido derivative (XXXIV).
WO 0202500
The condensation of benzaldehyde (I) with ethyl isovalerate (II) by means of hexyl lithium and DIA in THF gives the hydroxyester (III), which is acylated with Ac2O and DMAP in THF to yield the acetoxy derivate (IV). The elimination reaction in (IV) by means of t-BuOK in THF affords the unsaturated ester (V), which is hydrolyzed with KOH in ethanol to provide the unsaturated free acid (VI). Finally, this compound is enantioselectively reduced with H2 over several chiral Rh catalysts {[Rh(NBD)2BF4, [Rh(NBD)(OCOCF3)2], [Rh(NBD)Cl2], etc} to give the target intermediate 2(R)-isopropyl-3-[4-methoxy-3-(3-methoxypropoxy)phenyl]propionic acid (VII). (see scheme 26758001a, intermediate (VII)).
WO 0208172
The condensation of ethyl isovalerate (I) with 1,3-dichloropropene (II) by means of BuLi and DIA in THF gives 5-chloro-2-isopropyl-4-pentenoic acid ethyl ester (III), which is hydrolyzed with NaOH in ethanol to yield the corresponding racemic acid (IV). The optical resolution of (IV) is carried out by means of cinchonidine and TEA in THF to afford 5-chloro-2(S)-isopropyl-4-pentenoic acid (V), which can also be obtained by asymmetric synthesis as follows: Condensation of 4(S)-benzyl-3-(3-methylbutyryl)oxazolidin-2-one (VI) with 3-iodo-1-propenyl chloride (VII) by means of LiHMDS in THF gives 4(S)-benzyl-3-(2(S)-isopropyl-3-methylbutyryl)oxazolidin-2-one (VIII), which is hydrolyzed with LiOH in THF/water to afford the chiral pentanoic acid (V). The reaction of (V) with oxalyl chloride in toluene gives the corresponding acyl chloride (IX), which is treated with dimethylamine and pyridine in dichloromethane to yield the dimethylamide (X). The condensation of (X) with the chiral chloro derivative (XI) (obtained by reaction of the corresponding alcohol (XII) with CCl4 and trioctylphosphine) by means of Mg and 1,2-dibromoethane in THF affords the octenamide (XIII). The cyclization of (XIII) by means of phosphoric acid and simultaneous bromination with NBS in THF provides the chiral bromolactone (XIV), which is opened by means of dimethylamine and Et2AlCl in dichloromethane to give the chiral 5-bromo-4-hydroxy-2,7-diisopropyloctanamide (XV). The reaction of (XV) with acetic anhydride and pyridine in dichloromethane yields the acetoxy derivative (XVI), which is treated with LiN3 to afford the 5(S)-azido derivative (XVII).
The cyclization of (XVII) by means of TsOH in refluxing methanol gives the chiral lactone (XVIII), which is condensed with 3-amino-2,2-dimethylpropionamide (XIX) by means of TEA and 2-hydroxypyridine at 90 C to yield the corresponding amide (XX). Finally, the azido group of (XX) is reduced with H2 over Pd/C in tert-butyl methyl ether to afford the target Aliskiren.
WO 0202508
The condensation of the chiral chloro derivative (I) with 5-chloro-[2(S)-isopropyl]-4-pentanoic acid methyl ester (II) by means of Mg and dibromoethane in THF gives the chiral octenoic ester (III) which is converted to the corresponding acid (IV) by means of LiOH in THF/methanol/water. The reaction of (IV) with NBS in dichloromethane yields the bromolactone (V), which is treated with LiOH in isopropanol to yield the epoxide (VI). This compound, without isolation, is treated with HCl in the same solvent to afford the chiral hydroxylactone (VII). The reaction of the OH group of (VII) with MsCl and pyridine in toluene provides the mesylate (VIII), which is treated with NaN3 in hot 1,3-dimethylperhydropyrimidin-2-one to give the azido derivative (IX). The condensation of (IX) with 3-amino-2,2-dimethylpropionamide (X) by means of 2-hydroxypyridine in hot TEA yields the carboxamide (XI). Finally, the azido group of (XI) is reduced with H2 over Pd/C in tert-butyl methyl ether to provide the target Aliskiren.
Tetrahedron Lett 2000,41(51),10085
The intermediate gamma-butyrolactone (XXVIII) has been obtained as follows: Allylation of the imidazolidinone intermediate (V) with allyl bromide (XXI) and LiHMDS in THF gives the chiral intermediate (XXII), which by dihydroxylation and cleavage of the chiral auxiliary with OsO4 and NMMO in tert-butanol/acetone/water yields the lactone alcohol (XXIII). Oxidation of (XXIII) with NaIO4 and RuCl3 in CCl4/acetonitrile/water affords the carboxylic acid (XXIV), which by treatment with (COCl)2 in toluene provides the acyl chloride (XXV). Esterification of (XXV) with benzyl alcohol gives the corresponding benzyl ester as a diastereomeric mixture, from which the desired isomer (XXVI) is separated by flash chromatography. Hydrogenolysis of the benzyl ester (XXVI) with H2 over Pd/C in ethyl acetate yields the carboxylic acid (XXVII), which is treated with oxalyl chloride in toluene to afford the desired gamma-butyrolactone intermediate (XXVIII).
- Gradman A, Schmieder R, Lins R, Nussberger J, Chiang Y, Bedigian M (2005). “Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients”. Circulation 111 (8): 1012–8.doi:10.1161/01.CIR.0000156466.02908.ED. PMID 15723979.
- Straessen JA, Li Y, and Richart T (2006). “Oral Renin Inhibitors”. Lancet 368 (9545): 1449–56. doi:10.1016/S0140-6736(06)69442-7. PMID 17055947.
- “First Hypertension Drug to Inhibit Kidney Enzyme Approved”. CBC. 2007-03-06. Retrieved 2007-03-14.[dead link]
- Healthzone.ca: Blood-pressure drug reviewed amid dangerous side effects
- Parving, Hans-Henrik; Barry M. Brenner, M.D., Ph.D., John J.V. McMurray, M.D., Dick de Zeeuw, M.D., Ph.D., Steven M. Haffner, M.D., Scott D. Solomon, M.D., Nish Chaturvedi, M.D., Frederik Persson, M.D., Akshay S. Desai, M.D., M.P.H., Maria Nicolai
- Alkylation of 3-hydroxy-4-methoxybenzyl alcohol (I) with 1-bromo-3-methoxypropane (II) gives ether (III). Subsequent conversion of benzyl alcohol (III) into bromide (IV) is carried out using bromotrimetylsilane. The chiral isovaleryloxazolidinone (V) is alkylated with bromide (IV) by means of LiHMDS to afford (VI), which is hydrolyzed to the (S)-2-aryl-2-isopropylpropionic acid (VII) by means of lithium peroxide. The reduction of acid (VII) to the corresponding alcohol with NaBH4/I2 reagent, followed by treatment with PPh3 and NBS, provides bromide (VIII). Alkylation of the chiral dimethoxydihydropyrazin (IX) with bromide (VIII) produces (X). Further hydrolysis of the pyrazine ring of (X) with HCl, followed by Boc protection of the resulting (S,S)-amino ester, yields compound (XI). Reduction of the ester group of (XI) with DIBAL gives aldehyde (XII). This compound is condensed with the Grignard reagent (XIII) to afford the diastereomeric mixture of amino alcohols (XIV). Treatment of mixture (XIV) with 2,2-dimethoxypropane (XV) and TsOH produces a mixture of oxazolidines, from which the required (S,S,S)-isomer (XVI) is isolated by flash chromatography. Hydrogenolitic deprotection of the benzyl ether of (XVI) gives alcohol (XVII).des, M.D., Alexia Richard, M.Sc., Zhihua Xiang, Ph.D., Patrick Brunel, M.D., and Marc A. Pfeffer, M.D., Ph.D. for the ALTITUDE Investigators (2012). “Cardiorenal End Points in a Trial of Aliskiren for Type 2 Diabetes”. NEJM 367 (23): 2204–13. doi:10.1056/NEJMoa1208799. PMID 23121378.
- J “Chemistry & Biology : Structure-based drug design: the discovery of novel nonpeptide orally active inhibitors of human renin”. ScienceDirect. Retrieved 2010-01-20.
- Baldwin CM, Plosker GL.[1]. doi:10.2165/00003495-200969070-00004. Drugs 2009; 69(7):833-841.
- Ingelfinger JR (June 2008). “Aliskiren and dual therapy in type 2 diabetes mellitus”. N. Engl. J. Med. 358 (23): 2503–5.doi:10.1056/NEJMe0803375. PMID 18525047.
- PharmaXChange: Direct Renin Inhibitors as Antihypertensive Drugs
- Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. “Aliskiren Combined with Losartan in Type 2 Diabetes and Nephropathy,” N Engl J Med 2008;358:2433-46.
- Drugs.com: Tekturna
- Cardiorenal end points in a trial of aliskiren for type 2 diabetes, N Engl J MED. 2012;367(23):2204-2213
- European Medicines Agency recommends new contraindications and warnings for aliskiren-containing medicines.
- Prescribing Information for Tekturna
- aliskiren at the US National Library of Medicine Medical Subject Headings (MeSH)
- Chemical synthesis
Drugs Fut2001, 26, (12): 1139
Tetrahedron Lett 2001, 42: 4819-23.
Tetrahedron Lett2000, 41, (51): 10085
EP 0678500; EP 0678503; JP 1996053434; JP 1996081430; US 5559111; US 5627182; US 5646143, WO 0109079; WO 0109083
 | |
| SYSTEMATIC (IUPAC) NAME | |
|---|---|
| (2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethylethyl)-4-hydroxy-7-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-8-methyl-2-(propan-2-yl)nonanamide | |
| CLINICAL DATA | |
| AHFS/DRUGS.COM | monograph |
| MEDLINEPLUS | a607039 |
| LICENCE DATA | EMA:Link, US FDA:link |
| PREGNANCY CATEGORY |
|
| LEGAL STATUS | |
| ROUTES OF ADMINISTRATION | PO (oral) |
| PHARMACOKINETIC DATA | |
| BIOAVAILABILITY | Low (approximately 2.5%) |
| METABOLISM | Hepatic, CYP3A4-mediated |
| BIOLOGICAL HALF-LIFE | 24 hours |
| EXCRETION | Renal |
| IDENTIFIERS | |
| CAS NUMBER | 173334-57-1 |
| ATC CODE | C09XA02 C09XA52 (with HCT) |
| PUBCHEM | CID: 5493444 |
| IUPHAR/BPS | 4812 |
| DRUGBANK | DB01258 |
| CHEMSPIDER | 4591452 |
| UNII | 502FWN4Q32 |
| KEGG | D03208 |
| CHEBI | CHEBI:601027 |
| CHEMBL | CHEMBL1639 |
| CHEMICAL DATA | |
| FORMULA | C30H53N3O6 |
| MOLECULAR MASS | 551.758 g/mol |
////
O=C(N)C(C)(C)CNC(=O)[C@H](C(C)C)C[C@H](O)[C@@H](N)C[C@@H](C(C)C)Cc1cc(OCCCOC)c(OC)cc1