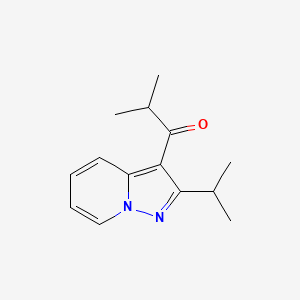
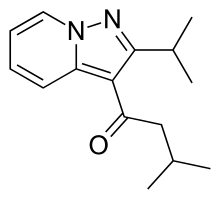
GCC-4401C ( GC-2107), Nokxaban
In phase 1 for treating thrombosis
5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene-2-carboxamide methanesulfonate
5-chloro-N-[[3-[4-(5,6-dihydro-2H-1,2,4-triazin-1-yl)phenyl]-2-oxo-1,3-oxazolidin-5-yl]methyl]thiophene-2-carboxamide
CB02-0133; GC-2107; GC4401; GCC-2107; GCC-4401; GCC-4401C; I Fxa – LegoChem Biosciences; LCB02-0133; Nokxaban
WO2010002115; LegoChem Bioscience INNOVATOR
| Green Cross Corporation, Legochem Bioscience Ltd. |
DEVELOPER
CAS NO FREE FORM

CAS 1159610-29-3, 159610-29-3, C18 H18 Cl N5 O3 S
2-Thiophenecarboxamide, 5-chloro-N-[[(5S)-3-[4-(5,6-dihydro-1,2,4-triazin-1(2H)-yl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-
Molecular Formula: C18H18ClN5O3S Molecular Weight: 419.88522 g/mol
METHANE SULFONATE

CAS 1261138-12-8, C18 H18 Cl N5 O3 S . C H4 O3 S,
2-Thiophenecarboxamide, 5-chloro-N-[[(5S)-3-[4-(5,6-dihydro-1,2,4-triazin-1(2H)-yl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-, methanesulfonate (1:1)
HYDROCHLORIDE
CAS 1261138-08-2., C18 H18 Cl N5 O3 S . Cl H, 2-Thiophenecarboxamide, 5-chloro-N-[[(5S)-3-[4-(5,6-dihydro-1,2,4-triazin-1(2H)-yl)phenyl]-2-oxo-5-oxazolidinyl]methyl]-, hydrochloride (1:1)

SUMMARY
- 09 Jan 2015GC 2107 is available for licensing as of 09 Jan 2015. http://www.greencross.com
- 01 May 2014Green Cross Corporation completes a phase I trial in Healthy volunteers in USA (NCT01954238)
- 26 Sep 2013Green Cross initiates enrolment in a phase I trial in Healthy volunteers in USA (NCT01954238)
Green Cross Corp in collaboration with LegoChem Bioscience, is developing GCC-4401C ( phase I), for treating thrombosis including venous thromboembolism
Development and Market Objectives
Green Cross Corporation is developing an orally available
direct Factor Xa inhibitor, GCC-4401C, which has shown an excellent
safety profile during Phase I clinical study. After completion of Phase
II and III studies for the prevention of venous thromboembolism (VTE) on
hip or knee replacement surgery patients, we will explore additional
indications for the treatment of acute coronary syndromes and the
prevention of stroke in patients with atrial fibrillation.
Unmet Medical Need & Target Patients
GCC-4401C may prove its greatest impact in providing a
much-needed and attractive alternative to warfarin in various
indications. Prophylaxis of deep vein thrombosis (DVT), which may lead
to pulmonary embolism in patients undergoing hip or knee arthroplasty,
is considered to be a primary unmet medical need. It is the most common
cause for rehospitalisation in this patient group. Each year in the
United States, between 350,000 and 600,000 people experience a blood
clot in the legs or in the lungs. The US and European hip and knee
implant markets are the two largest, accounting for nearly 80 percent of
total procedures conducted worldwide. The 2005 revenues for hip and
knee implants in the US and Europe were $6.5 billion. Demand driven by
an aging population and an increasing number of younger patients are
contributing to the continuous growth of hip and knee replacement
procedures.
Thromboembolism involving arterial or venous circulation is a common
cause of morbidity and mortality. As an anticoagulation therapy, heparin
and Vitamin K antagonists (VKAs) such as warfarin have been used in
clinical settings for more than 50 years, but both are associated with
several limitations requiring frequent coagulation monitoring due
to unpredictable effects of anticoagulant . Therefore, there is an
urgent need for novel, oral agents with a predictable anticoagulant
action. The greatest unmet medical need in anticoagulation therapy is to
find a replacement for VKAs for long-term therapy, particularly stroke
prevention in patients with atrial fibrillation (a heart rhythm
disorder). Recently, Factor Xa has emerged as an attractive target for
novel anticoagulants and a number of Factor Xa inhibitors are currently
under development as oral anticoagulants for long-term use.A major unmet medical need is for direct FXa inhibitors that are simpler to administer than VKAs, with fewer strokes and less intracranial bleeding compared with warfarin and less bleeding yet similar or better efficacy with a lower-dose regimen. In addition, the availability of simple, fixed-dose, unmonitored therapies should increase the use of direct FXa inhibitor therapy in patients with atrial fibrillation at risk for stroke.
Status
Phase I Clinical Study
To investigate the safety and tolerability of single doses of
GCC-4401C in healthy male subjects, a Phase Ia study (GCC-4401C-101) was
recently conducted at Quintiles in the United States under the
conditions of randomized, double-blind, placebo-controlled, and single
ascending dose. Forty eight healthy male subjects were enrolled in 6
cohorts and administered at 6 dose-escalation levels up to 80
mg/subject. GCC-4401C was well-tolerated without any significant adverse
events, and was detected in blood plasma dose-proportionally across the
dose range of 2.5 mg to 80 mg per patient. The pharmacodynamic
variables were also statistically correlated with GCC-4401C plasma
concentrations.We plan to characterize the safety, tolerability, pharmacokinetics and pharmacodynamics of multiple doses of GCC-4401C in healthy male subjects based on the safety margins of the SAD study. An appropriate dose and dosing regimen of oral GCC-4401C from subsequent clinical trials on VTE patients are expected to be identified. The Phase 1b study will be completed with Global CRO in the US in 3Q, 2014.
Intellectual Property
Material patent for GCC-4401C, covering a wide range of
chemical structures, was awarded in early 2008 within S. Korea, followed
by its production method patent in early 2011. Moreover, patent
applications for both material and production method, are in progress in
21 and 5 overseas countries including the US, respectively.
– KR811865 : Pyrimidinone derivatives or pyridazinone derivatives for inhibition of factor VIIa activity
– KR109594 : FXa inhibitors with cyclic amidines as P4 subunit, processes for their preparations, and pharmaceutical compositions and derivatives thereof
– KR898361 : FXa inhibitors with cyclic amidoxime or cyclic amidrazone as P4 subunit, processes for their preparations, and pharmaceutical compositions and derivatives thereof
– KR1037051 : Method for preparing of (S)-5-chloro-N-((3-(4-(5,6-dihydro-4H-1,2,4-oxadiazin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl)thiophene-2-carboxamide derivatives
– KR1037052 : Method for preparing 5-chloro-N-(((5S)-2-oxo-3-(4-(5,6-dihydro-1,2,4-triazin-1(4H)-yl)phenyl)-1,3-oxazolidin-5-yl)methyl)thiophen-2-carboxamide derivatives, and their intermediates
– PCT/KR2010/004420 : Method for preparing (S)-5-chloro-N-((3-(4-(5,6-dihydro-4H-1,2,4-oxadiazin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl)thiophene-2-carboxamide derivatives
– PCT/KR2010/004421 : Method for preparing 5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene-2-carboxamide derivative and intermediate used therein
– KR811865 : Pyrimidinone derivatives or pyridazinone derivatives for inhibition of factor VIIa activity
– KR109594 : FXa inhibitors with cyclic amidines as P4 subunit, processes for their preparations, and pharmaceutical compositions and derivatives thereof
– KR898361 : FXa inhibitors with cyclic amidoxime or cyclic amidrazone as P4 subunit, processes for their preparations, and pharmaceutical compositions and derivatives thereof
– KR1037051 : Method for preparing of (S)-5-chloro-N-((3-(4-(5,6-dihydro-4H-1,2,4-oxadiazin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl)thiophene-2-carboxamide derivatives
– KR1037052 : Method for preparing 5-chloro-N-(((5S)-2-oxo-3-(4-(5,6-dihydro-1,2,4-triazin-1(4H)-yl)phenyl)-1,3-oxazolidin-5-yl)methyl)thiophen-2-carboxamide derivatives, and their intermediates
– PCT/KR2010/004420 : Method for preparing (S)-5-chloro-N-((3-(4-(5,6-dihydro-4H-1,2,4-oxadiazin-3-yl)phenyl)-2-oxooxazolidin-5-yl)methyl)thiophene-2-carboxamide derivatives
– PCT/KR2010/004421 : Method for preparing 5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene-2-carboxamide derivative and intermediate used therein
Competitive Advantages
GCC-4401C has been specifically designed for chronic,
once-a-day treatment. It has a half-life that supports true, once-daily
dosing and a low peak-to-trough drug concentration ratio that minimizes
anticoagulant variability. Since GCC-4401C has an excellent aqueous
solubility, there has been potential for the development of both po and
iv formulations. Data from comparative efficacy studies in animals have
also demonstrated the superiority of GCC-4401C against other direct FXa
inhibitors with less bleeding effects. From the recent Phase Ia clinical
study, GCC-4401C did not show any significant sign of adverse
events. PK parameters and PD markers were predictable
dose-proportionally across the all dose ranges. GCC-4401C is expected to
show excellent safety profiles, less bleeding and less liver toxicity
through human clinical studies.
Contact & Company Overview
-
Company Name :
Green Cross Corporation -
Homepage :
-
Contact Person :
Hyoung Geun Beak, Deputy General Manager, Business -
E-mail :
-
Contact :
+82-31-260-9337
PATENT
WO 2016010178GREEN CROSS CORPORATION [KR/KR]; 107, Ihyeon-ro 30beon-gil, Giheung-gu, Yongin-si, Gyeonggi-do 446-770 (KR).
LEGOCHEM BIOSCIENCES, INC. [KR/KR]; 8-26, Munpyeongseo-ro, Daedeok-gu, Daejeon 306-220 (KR)
The present invention relates to a novel crystalline form of 5-chloro-N-({(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[1,2,4]triazin-1-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene-2-carboxamide methanesulfonate and a pharmaceutical composition containing the same. The novel crystalline form of a compound according to the present invention exhibits excellent stability even in high-temperature and humidity environments, and thus can be favorably used to prevent or treat diseases, such as thrombosis, myocardial infarction, atherosclerosis, inflammation, stroke, angina pectoris, restenosis after angioplasty, and thromboembolism.
According to the present invention 5-chloro -N – ({(5 S) -2- oxo-3- [4- (5,6-dihydro the -4H- [1, 2, 4] triazine-1-yl) phenyl] -1, 3-oxazolidin-5-yl} methyl) thiophene-2-mid copy methane sulfonic acid salt (hereinafter referred to as a new crystal form has excellent solubility referred to) in “GCO4401C”, Ko Un and wet environments It is excellent in stability.
Novel crystalline forms of GCC-4401C of the present invention, the organic solvent under reduced pressure crystallization method, a cooling crystallization method or solvent-can be easily obtained by the anti-solvent crystallization process.
Ateumyeo GCC-4401C is used as a reaction raw material can be prepared according to the procedure described in PCT Publication No. W02011 / 005029 No., dissolving the starting compound in an organic solvent the semi-adding a solvent after filtration to determine the resulting mixture was cooled and then dried to give the novel crystalline form can be a compound according to the invention.
PATENT
http://www.google.com/patents/WO2011005029A2?cl=en
5-Chloro-N-( {(5S)-2-oxo-3-[4-(5,6-dihydro-4H-[ 1 ,2,4]triazin- 1-yl)phenyl]-l,3-oxazolidin-5-yl}-methyl)thiophene-2-carboxamide of formula (A) has been known as an inhibitor of blood coagulation factor Xa and used for treating and preventing thrombosis, myocardial infarction, arteriosclerosis, inflammation, stroke, angina pectoris, recurrent stricture after angioplasty, and thromboembolism such as intermittent claudication.

Korea Patent No. 2008-64178, whose application has been filed by the present invetors, discloses a use of the compound as an inhibitor of blood coagulation factor Xa and a preparation method thereof. The preparation method comprises the step of preparing a cyclic amidrazone starting from 4-nitroaniline, as shown in reaction scheme 1 :
Reaction Scheme 1

Specifically, the cyclic amidrazone (A) is prepared by the steps of: preparing the compound (B) using 4-nitroaniline; treating the compound (B) with a t-butoxycarbonyl amine protecting group to prepare the compound (C); introducing a nitroso group into the compound (C) using NaNO2, followed by reduction using zinc to prepare the compound (D); and treating the compound (D) successively with hydrochloric acid and an ortho-formate.
However, the above preparation method is complicated and gives a low yield of the compound (A) (e.g., a total yield of 9 %), and it also requires the use of a column chromatography purification step, which limits mass production of the cyclic amidrazone. In particular, the step for preparing the compound (D) from the compound (C) is required to use a harmful heavy metal-containg materal such as zinc amalgam which gives an unsatisfactorily low yield, and the isolation step of the compound (D) does not proceed easily.
Reaction Scheme 2

Reaction Scheme 3

Example 1: Preparation of Ethyl formimidate hydrochloride

To a solution of benzoyl chloride (1212 g, 8.62 mol, 1 eq) in anhydrous ether (5.8 L) was added dropwise a solution of formamide (388 g, 8.62 mol, 1 eq) in EtOH (396 g, 8.60 mol, 0.998 eq) at 0 °C for lhr. The mixture thus obtained was stirred at 0 °C for 30min. The solid was filtered off, washed with ether (3 L) and EA (3 L). The solid was dried under high vacuum.
Yield : 625 g (66%)
Example 1: 5-chloro-N-({(5S)-2-oxo-3-[(5,6-dihydro-lH-[l,2,4]triazin-4-yl)phenyl]-l,3-oxazolidin-5-yl}-methyl)-2-thiophene carboxamide hydrochloride
Step 1: Preparation of 2- [N-(4-nitro-phenyl)-hydrazino]-ethanol

l-Fluoro-4-nitrobenzene (7.1 g, 50 mmol) was dissolved in CH3CN (70 ml), 2-hydroxyethylhyrazine (purity: 90 %, Aldrich, 5.0 g, 66 mmol) and K2CO3 (7.6 g, 55 mmol) were added thereto. The suspension thus obtained was stirred for 4 hrs with reflux. The resulting orange-colored suspension was concentrated under reduced pressure (reflux condenser, 10 torr, 40 °C) and ethylacetate (EA, 90 ml) and water (18 ml) were added thereto. The resulting mixture was stirred strongly at r.t. for 10 min. The organic layer was extracted and washed with the saturated brine (10 ml). The resulting solution was cooled to 10 °C and 48 % HBr solution (3.7 ml) was added thereto dropwise with stirring. The pale yellow colored solid thus obtained was filtered off and dried under high vacuum (1 torr, 40 “C) to obtain the title compound as an intermediate.
Yield: 7.1 g (51 %).
TLC : Rf= 0.62 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR (600 MHz, DMSO-J6) δ 8.17 (d, J = 9.0 Hz, 2H), 7.12 (d, J = 9.0 Hz, 2H), 3.82 (t, J= 5.4 Hz, 2H), 3.69 (t, J= 5.4 Hz, 2H)
LCMS: 198 (M+H+) (C8H11N3O3)
Step 2: Preparation of l-bromo-2-[N-(4-nitro-phenyl)-hydrazino] -ethane

The compound obtained in Step 1 (38.9 g, 0.140 mol) was suspended in anhydrous 1 ,2-dimethoxyethane (585 ml). The resultant suspension was cooled to 0 °C and PBr3 (15.9 ml, 0.168 mol) was added thereto dropwise for 30 min. The mixture thus obtained was stirred at 60 °C for 4 hrs. The pale yellow colored solution thus obtained was concentrated under reduced pressure (reflux condenser, 10 torr, 45 °C). The resultant residue (oil) was suspended with water (150 ml) and stirred. Aq. sat’d NaHCO3 solution (150 m) was added to the resultant suspension to be pH 4. The resulting mixture was stirred for 30 min to precipitate the pale yellow colored precipitates. The precipitates were filtered off and washed with water (100 ml). The resulting solid was mixed with water (100 ml), aq. sat’d NaHCO3 solution (70 ml) and CH2Cl2 (500 ml). The resulting mixture was stirred for 10 min and stood to separate organic and aqueous layers. The organic layer was dried over 20 g of MgSO4 and filtered off. The resulting filterate was concentrated under reduced pressure (reflux condenser, 10 torr, 40 °C) to obtain the title compound as a pale yellow solid.
Yield : 31.3 g (86 %)
TLC : Rf= 0.91 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR (600 MHz, CDCl3) δ 8.14 (d, J = 10.2 Hz, 2H), 6.92 (d, J= 10.2 Hz, 2H), 4.00 (t, J= 7.2 Hz, 2H), 3.65 (t, J= 7.2 Hz, 2H)
LCMS: 261 (M+H+) (C8H10BrN3O2)
Step 3: Preparation of 4-(5,6-dihydro-4H-[l,2,4]triazin-l-yl)-l-nitrobenzene

The compound obtained in Step 2 (13.0 g, 50.0 mmol) was completely dissolved in anhydrous 1,2-dimethoxyethane (200 ml) which is prepared by mixing 1,2-dimethoxyethane (purity: 99 %, Junsei Co. Ltd) with an desired amount of molecular sieve 4A and standing for 5 hrs or more with stirring at times. Ethyl formimidate HCl salt (5.8 g, 52.5 mmol) was added thereto. The suspension thus obtained was stirred at 25 °C for 10 min. Anhydrous sodium acetate (NaOAc, 8.6 g, 105 mmol) was added thereto and stirred for 15 hrs with reflux. The orange colored suspension thus obtained was concentrated under reduced pressure (10 torr, 50 “C). The orange colored residue thus obtained was mixed with IN HCl (140 ml), EA (50 ml) and hexane (100 ml), and stirred at r.t for 10 min. A small amount of insoluble suspended solids was remained in aqueous layer and filtered off. The resulting aqueous layer was washed with a mixture of EA (30 ml) and hexane (60 ml). 12 g of sodium carbonate was added to the resulting solution to be pH 8.5. The orange colored solid thus obtained was filtered off under reduced pressure, washed with water (15 ml) and dried under vacuum to obtain the title compound .
Yield : 7.7 g (75 %).
TLC : R/= 0.45 (EA/MeOH/AcOH = 20/1/0.5)
HPLC : R, = 8.65 (Gradient A), purity 91.1%
1H NMR (400 MHz, DMSO-^6) δ 8.03 (d, J= 9.6 Hz, 2H), 7.16 (d, J = 9.6 Hz, 2H), 7.12 (br s, IH), 7.01 (d, J= 4.0 Hz, 2H), 3.77 (t, J= 5.2 Hz, 2H), 3.43-3.40 (m, 2H)
LCMS: 207 (M+H+) (C9H10N4O2)
Step 4: Preparation of 4-(5,6-dihydro-4-t-butoxycarbonyl-[l,2,4]triazin-l-yl)-1-nitrobenzene
To the orange colored suspension prepared by suspending the compound obtained in Step 3 (12.4 g, 60 mmol) in tetrahydrofurane (THF, 200 ml), 4-dimethylaminopyridine (DMAP, 0.367 g, 3 mmol) and di-tert-butyl dicarbonate
(BoC2O, 19.6 g, 90 mmol) were added and stirred with reflux for 1.5 hrs. The yellow colored suspension thus obtained was concentrated under reduced pressure
(reflux condenser, 10 torr, 40 °C) to remove the solvent. The resulting yellow colored residue was completely dissolved in CH2Cl2 (700 ml) and washed with IN HCl (700 ml). The organic layer was extracted, dried over 25 g of MgSO4, and concentrated under reduced pressure (condenser, 10 torr, 40 °C). The resultant yellow colored residue was dissolved in cyclohexane (250 ml) and stirred strongly at r.t. for 30 min. The resulting mixture was concentrated under reduced pressure to obtain yellow colored solids. The solids were dried (1 torr, 50 °C ) to obtain a disried compound.
Yield: 15.6 g (85 %)
TLC : R/= 0.93 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR (600 MHz, DMSO-J6) δ 8.14 (d, J= 9.6 Hz, 2H), 7.62 (br s, IH), 7.30 (d, J = 9.6 Hz, 2H), 3.89 (br s, 2H), 3.79 (br s, 2H), 1.50 (s, 9H)
LCMS: 307 (M+H+) (C14H18N4O4)
Step 5: Preparation of 4-(5,6-dihydro-4-t-butoxycarbonyl-[l,2,4]triazin-l-yl)aniline
To the yellow colored suspension prepared by suspending the compound obtained in Step 4 (19.9 g; 65 mmol) in methanol (200 ml), 10 % palladium on carbon (4.0 g) was added. The resulting mixture was subjected to vacuum outgassing and stirred at r.t., for 2 hrs in the flask connected with hydrogen bollum. The resulting mixture was filtered through celite 545 under redued pressure to remove the palladium on carbon. The fϊlterate was concentrated under reduced pressure (reflux condenser, 10 torr, 40 °C). The resulting pale brown colored residue was dissolved in isopropylalcohol (140 ml) and refluxed to dissolve completely. The resulting solution was stood at 0 °C for 2 hrs to cool, stirred for 30 min and filtered off under redued pressure. The resulting ivory crystalline solid was dried in vacuo to obtain the title compound (15.8 g, 88 %).
TLC : Rf= 0.38 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR (400 MHz, DMSO-(I6) δ 7.34 (br s, IH), 6.91 (d, J = 12.0 Hz, 2H), 6.51 (d, J = 12.0 Hz, 2H), 6.64 (br s, 2H), 3.74 (br s, 2H), 3.41 (br s, 2H), 1.48 (s, 9H)
LCMS: 277 (M+H+) (C14H20N4O2)
Step 6: Preparation of N-(3-(5,6-dihydro-4-t-butoxycarbonyl-[l,2,4]triazin-l-yI)anilino-(2R)-2-hydroxypropyI)-5-chloro-2-thiophene carboxamide

The compound obtained in Step 5 (19.3 g, 70 mmol) and 5-chloro-N-(((S)-oxiran-2-yl)methyl)thiophene-2-carboxamide (19.1 g, 88 mmol) were suspended in isobutyl alcohol (350 ml) and stirred for 18 hrs with reflux. The dark blue colored solution thus obtained was concentrated under reduced pressure (reflux condenser, 10 torr, 50 °C). To the yellow solid residue thus obrained, ethylacetate (200 ml) was added and the resulting mixture was stirred at r.t. for 30 min and further stirred strongly at 0 °C for 30 min. The suspended solid thus obtained was filtered off under reduced pressure and dried in vaccum (1 torr, 50 °C ) to obtain the title compound as ivory crude.
Yield : 25.9 g (75 %)
TLC : R/= 0.34 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR of a crude sample (600 MHz, DMSO-</6) δ 8.62 (t, J = 5.4 Hz, IH), 7.69 (d, J = 3.6 Hz, IH), 7.36 (br s, IH), 7.18 (d, J = 4.2 Hz, IH), 6.95 (d, J = 9.0 Hz, 2H), 6.54 (d, J = 9.0 Hz, 2H), 5.10 (t, J = 6.6 Hz, IH), 5.05 (d, J = 5.4 Hz, IH), 3.81-3.75 (m, 3H), 3.44 (br s, 2H), 3.37-3.34 (m, IH), 3.25-3.21 (m, IH), 3.08-3.04 (m, IH), 2.94-2.89 (m, IH), 1.48 (s, 9H)
LCMS: 494 (M+H+) (C22H28ClN5O4S)
Step 7: Preparation of 5-chloro-N-({(5S)-2-oxo-3-[(5,6-dihydro-4-t-butoxycarbonyl-[l,2,4]triazin-l-yl)phenyl]-l,3-oxazolidin-5-yI}-methyl)-2-thiophene carboxamide

The compound obtained in Step 6 (25.2 g, 51 mmol) was completely dissolved in THF (325 ml), and Ll’-carbonyldiimidazole (10.8 g, 66 mmol) and DMAP (0.31 mg, 2.6 mmol) were added thereto. The resulting mixture was stirred with reflux for 18 hrs. The resulting pale yellow colored suspension was cooled to r.t, concentrated under reduced pressure and dried in vacuo (1 torr, 50 °C) to obtain the title compound as an ivory solid.
Yield : 23.3 g (88 %)
TLC : R/= 0.75 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR (400 MHz, DMSO-J6) δ 8.97 (t, J = 5.4 Hz, IH), 7.69 (d, J= 4.2 Hz, IH), 7.43 (br s, IH), 7.41 (d, J = 9.0 Hz, 2H), 7.20 (d, J = 4.2 Hz, IH), 7.19 (d, J= 9.0 Hz, 2H), 4.82-4.77 (m, IH), 4.12 (t, J= 9.0 Hz, IH), 3.80-3.78 (m, 3H), 3.62 (br s, 2H), 3.59 (t, J= 6.0 Hz, 2H), 1.49 (s, 9H)
LCMS: 520 (M+H+) (C23H26ClN5O5S)
Step 8: Preparation of 5-chloro-N-({(5S)-2-oxo-3-[(5,6-dihydro-4H-[l,2,4]triazin-l-yl)phenyl]-l,3-oxazolidin-5-yl}-methyl)-2-thiophene
carboxamide hydrochloride

The compound obtained in Step 7 (16.1 g, 31 mmol) was completely
dissolved in THF (193 ml), 3N HCl (193 ml) was added thereto. The resulting solution was stirred with reflux for 1 hr. The white suspension thus obtained was cooled tq r.t, concentrated under reduced pressure and dried in vacuo (1 torr, 40 °C ) to obtain the title compound as a white solid.
Yield : 13.4 g (95 %)
TLC : R/= 0.82 (MC/MeOH/AcOH = 10/1/0.5)
HPLC : R, = 12.39 (Gradient A), purity 99.5%
1H NMR (600 MHz, OMSO-d6) δ 12.12 (br s, IH), 10.20 (br s, IH), 9.08
(t, J = 6.0 Hz, IH), 8.60 (d, J = 5.2 Hz, IH), 7.74 (d, J= 4.2 Hz, IH), 7.53 (d, J = 9.0 Hz, 2H), 7.20 (d, J= 4.2 Hz, IH), 7.13 (d, J= 9.0 Hz, 2H), 4.85-4.81 (m, IH),
4.15 (t, J = 8.8 Hz, IH), 3.85 (dd, J = 6.0, 9.2 Hz, IH), 3.66 (t, J = 4.8 Hz, 2H),
3.63-3.56 (m, 2H), 3.19 (br s, 2H)
LCMS: 420 (M+H+) (C18H18ClN5O3S)
Example 2: Preparation of 5-chloro-N-({(5S)-2-oxo-3-[(5,6-dihydro-4H-[l,2,4]triazin-l-yI)phenyl]-l,3-oxazolidin-5-yl}-methyI)-2-thiophene
carboxamide

The HCl salt obtained in Example 1 (6.9 g, 15 mmol) was completely dissolved in 33 % methanol aqueous solution (1.1 L) and heated to 50 °C while stirring. To the resulting colorlessness solution, 0.6M aq. Na2CO3 solution (25 ml) was added and the white suspension thus obtained was stood at 0 °C for 0.5 hr to cool. The white solid thus obtained was concentrated under reduced pressure, wished with H2O (150 ml) and dried in vacuo (1 torr, 40 “C) to obtain the title compound (yield: 5.5 g, 87 %). The title compound was dissolved in methanol (330 ml) and stirred with reflux. The pale yellow colored solution thus obtained was stood at 0 °C for 2 hrs to cool. The resulting white solid was concentrated under reduced pressure, washed with methanol (10 ml), and dried in vacuo (1 torr, 40 “C) to obtain a crystal of the title compound (yield: 5.0 g, 80 %).
HPLC : R, = 12.37 (Gradient A), purity 99.7 %
1H NMR (400 MHz, DMSO-^6) δ 8.97 (t, J = 6.0 Hz, IH), 7.69 (d, J = 4.0 Hz, IH), 7.32 (d, J = 9.2 Hz, 2H), 7.20 (d, J = 4.0 Hz, IH), 7.12 (d, J = 9.2 Hz, 2H), 6.79 (d, J = 4.0 Hz, IH), 6.52 (br s, IH), 4.80-4.75 (m, IH), 4.10 (t, J = 8.8 Hz, IH), 3.77 (dd, J= 6.0, 9.2 Hz, IH), 3.58 (t, J= 5.6 Hz, 2H), 3.33 (s, 4H)
LCMS: 420 (M+H+) (C18H18ClN5O3S)
Example 3: Preparation of 5-chloro-N-({(5S)-2-oxo-3-[(5,6-dihydro-4H-[l,2,4]triazin-l-yl)phenyl]-l,3-oxazolidin-5-yI}-methyI)-2-thiophene carboxamide methane sulfonate

To the compound obtained in Example 2 (3.3 g, 7.9 mmol), a mixture solution of MeOH/CH2Cl2 (1/4 v/v, 70 ml) was added and stirred with reflux. The pale yellow colored solution thus obtained was cooled to 0 °C and methylsulfonic acid (0.56 ml, 8.6 mmol) was added thereto. The resulting mixture was concentrated under reduced pressure (reflux condenser, 10 torr, 40 °C) to obtain pale yellow foamy solid. To the resultant solid, absolute ethanol (20 ml) was added and the resulting mixture was stirred with reflux to dissolve solid clearly. The resulting solution was cooled to 0 °C to 2 hrs. The resulting white solid was concentrated under reduced pressure, washed with absolute EtOH (5 ml), and dried in vacuo (1 torr, 40 “C) to obtain a crystalline methane sulfonate.
Yield : 3.8 g (93 %)
HPLC : R, – 12.35 (Gradient A), purity 99.8%
1H NMR (400 MHz, DMSO-CZ6) δ 11.97 (br s, IH), 10.07 (br s, IH), 8.99
(t, J= 6.0 Hz, IH), 8.59 (U1 J= 6.0 Hz, IH), 7.70 (d, J= 4.0 Hz, IH), 7.53 (d, J =
9.2 Hz, 2H), 7.20 (d, J= 4.0 Hz, IH), 7.13 (d, J= 9.2 Hz, 2H), 4.86-4.80 (m, IH),
4.16 (t, J = 9.2 Hz, IH), 3.82 (dd, J = 6.0, 9.2 Hz, IH), 3.67 (m, 2H), 3.60 (t, J = 5.6 Hz, 2H), 3.20 (br s, 2H), 2.31 (s, 3H)
LCMS: 420 (M+H+)(C18H18ClN5O3S)
Example 4: (S)-5-chloro-N-((3-(4-(5,6-dihydro-l,2,4-triazin-l(4H)-yl)phenyI)-2-oxooxazolidin-5-yl)methyl)thiophene-2-carboxamide methane sulfonate
Step 1: Preparation of (2-[N-(4-nitro-phenyl)-hydrazinyl]-ethanol) hydrobromide

l-Flouro-4-nitrobenzene (428 g, 3.03 mol, Aldrich Fl 1204) was dissolved in CH3CN (4.3 L), and 2 -hydroxy ethylhyrazine (300 g, 3.94 mol, 1.3 eq, imported from China, >98 %) and K2CO3 (461 g, 3.34 mol, 1.1 eq, Aldrich
347825) were added thereto. The mixture thus obtained was stirred at 80 °C for
19 hrs. The mixture was cooled to r.t. and evaporated to remove solvent. The residue was dissolved with EA (1.5 L) and H2O (1 L). The organic layer was extracted and washed with H2O (500 mL) and brine (200 mL). The extracted
EA layer was cooled to 0 °C and 48 % HBr solution (360 mL, Aldrich 244260) was added thereto dropwise at 0 °C with stirring. The resultant mixture was stirred at 0 °C for 1 hr. The solid thus obtained was filtered off and washed with
EA (5 L). The obtained solid was dried under high vacuum to obtain the title compound.
Yield : 531 g (63 %)
TLC : Rf= 0.62 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR (400 MHz, OMSO-d6) δ 7.94 (d, J = 9.6 Hz, 2H), 7.12 (br s, 2H), 6.63
5.8 Hz, 2H) LCMS: 198 (M+H+) (C8H11N3O3)
Step 2: Preparation of l-bromo-2-[N-(4-nitro-phenyl)-hydrazino]-ethane

The compound obtained in Step 1 (531 g, 1.90 mol) was suspended in
anhydrous 1,2-dimethoxyethane (4.5 L). The resultant suspension was cooled to 0 °C and PBr3 (220 niL, 2.29 mol, 1.2 eq, Aldrich 256536) was added thereto dropwise at 0 °C . The mixture thus obtained was warmed up to r.t. and stirred at 6O 0C for l5 hrs.
The mixture was cooled to r.t., and filtered off to remove remained insoluble solid. The filter cake thus obtained was washed with 1,2- dimethoxyethane (700 mL) and the filtrate was concentrated in vacuo. The resultant residue was suspended with H2O (2.5 L), stirred and cooled to 0 °C . Aq. 2N NaOH solution (1.7 L) was added thereto at 0°C to neutralize the suspension mixture (pH 6-7). The solid was filtered off and washed with H2O (5 L). The filtered solid was air-dried for 5 hrs.
The air-dried solid was dissolved with CH2Cl2 (3 L), and aq. sat’d
NaHCO3 solution (1.5 L) and H2O (700 mL) were added thereto. The resultant
– mixture was stirred for 15 min and stood to separate organic and aqueous layers. Insoluble solid which was not dissolved in organic layer and H2O was remained in the mixture. The mixture was filtered off to remove insoluble solid and the filter cake was washed with CH2Cl2 (700 mL). The organic layer was extracted, dried over MgSO4, filtered off, and concentrated in vacuo. The resultant solid was dried under high vacuum to obtain the title compound.
Yield : 383 g (77% : When product was dissolved in CDCl3 to check the
1H NMR spectroscopy, insoluble solid was stilled remained in CDCl3)
TLC : Rf= 0.91 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR (400 MHz, CDCl3) δ 8.12 (d, J = 9.6 Hz, 2H), 6.92 (d, J = 9.2 Hz, 2H), 4.00 (t, J = 6.6 Hz, 2H), 3.65 (t, J = 6.6 Hz, 2H)
LCMS: 261 (M+H+) (C8H10BrN3O2)
Step 3: Preparation of 4-(5,6-dihydro-4H-[l,2,4]triazin-l-yl)-l-nitrobenzene
Ethyl formimidate HCI, NaOAc
1 ,2-dimethoxyethane

The compound obtained in Step 2 (384 g, 1.48 mol) was dissolved in anhydrous 1,2-dimethoxyethane (4 L) and ethyl formimidate HCl salt (322 g, 2.94 mol, 2 eq) was added thereto at r.t. The resultant mixture was stirred at r.t. for 30 min. NaOAc (364 g, 4.44 mol, 3.0 eq, Aldrich 110191) was added to the mixture and the mixture was stirred at 75 °C for 15 hrs.
The mixture was cooled to r.t. and evaporated to remove solvent. The resultant residue was suspended in EA (2 L) and 1,2-dimethoxyethane (I L). Aq.
3N HCl solution (2.5 L) was added to the suspension. Insoluble solid was remained in resultant mixture. The solid was filtered off two times to remove insoluble solid. Ether (3 L) was added to the filtrate to separate organic and aqueous layers effectively. Aqueous layer was separated and washed with mixed organic solution (EA (1 L) + Hexane (500 mL)). The combined organic layer should be kept to recover the product.
(The treatment of aqueous layer)
The aqueous layer was cooled to 0 °C and aq. 6N NaOH solution (2.2 L) was added thereto slowly to basify the H2O layer (pH ~ 9). The resultant suspension was stirred at r.t. for 12 hrs. The solid was filtered off and washed with H2O (3 L) and dried under high vacuum.
(The treatment of combined organic layer)
The combined organic layer was concentrated in vacuo. The resultant residue was acidified with aq. 3N HCl solution (500 mL). Filtration was carried out to remove insoluble solid. The filtrate (H2O layer) thus obtained was washed with ether (700 mL X 2). The aqueous layer was stirred and cooled to 0 °C . Aq. 5N NaOH solution (1 L) was added to the cooled aqueous layer to basify (pH ~9). The mixture thus obtained was stirred at r.t. for 12 hrs. The solid thus obtained was filtered off and washed with H2O (1.5 L). The solid was dried under high vacuum to obtain the title compound.
Yield : 187 g (62 %)
TLC : Rf= 0.45 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR (400 MHz, DMSO-</6) δ 7.99 (d, J = 9.6 Hz, 2H), 7.16 (d, J =
9.6 Hz, 2H), 7.09 (br s, IH), 6.97 (d, J = 3.6 Hz, 2H), 3.73 (t, J = 5.0 Hz, 2H), 3.45-3.46 (m, 2H)
LCMS: 207 (M+H+) (C9H10N4O2)
Step 4: Preparation of 4-(5,6-dihydro-4-t-butoxycarbonyl-[l,2,4]triazin-l-yl)- 1-nitrobenzene
The compound obtained in Step 3 (187g, 0.907 mol) was suspended in anhydrous THF (2.2 L), and BoC2O (30Og, 1.36 mol, 1.5 eq, Aldrich 205249) and DMAP (6g, 0.045 mol, 0.05 eq, Aldrich 107700) were added thereto. The mixture thus obtained was stirred at 65 °C for 5 hrs.
The mixture was cooled to 0 °C . MeOH (1.5 L) was added to the mixture at 0 °C and stirred at 0 °C for 1 hr. The solid thus obtained was filtered off, washed with MeOH (750 niL) and dried under high vacuum.
Filtrate thus obtained was concentrated in vacuo. MeOH (1 L) was added to the resultant residue with stirring. The mixture thus obtained was stirred at r.t for 12 hrs. Solid thus obtained was filtered off, washed with MeOH (500 mL), and dried under high vacuum to obtain the title compound.
Yield : 182 g (65 %)
TLC : Rf= 0.93 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR (400 MHz, DMSO-J6) δ 8.17 (d, J= 9.6 Hz, 2H), 7.57 (br s, IH), 7.19 (d, J= 9.6 Hz, 2H), 3.93-3.86 (m, 2H), 3.83-3.745 (m, 2H), 1.56 (s, 9H)
LCMS: 307 (M+H+) (C14H18N4O4)
Step 5: Preparation of 4-(5,6-dihydro-4-t-butoxycarbonyl-[l,2,4]triazin-l-yl)aniline
The compound obtained in Step 4 (134 g, 438 mmol) was suspended in
MeOH (1.3 L) at r.t., and NH4Cl (12 g, 0.5 eq, Aldrich A4514) and Zn (15 g, 0.5 eq, Aldrich 209988) were added 6 times at intervals of 15 min at r.t. (total amounts Of NH4Cl = 73 g (1356 mmol, 3.1 eq) and total amounts of Zn = 88 g
(1356 mmol, 3.1 eq))
Temperature of the resultant mixture was risen gradually to 65 °C and the mixture was stirred at 65 °C for 12 hrs. The mixture was cooled to 40 °C and NH4Cl (12 g, 0.5 eq, Aldrich A4514) and Zn (15 g, 0.5 eq, Aldrich 209988) were added thereto. Temperature of the resultant mixture was risen gradually to 65 °C and the mixture was stirred at 65 “C for 1 hr.
The mixture was cooled to r.t. and filtered off through celite pad. The filter cake was washed with MeOH (700 mL) and THF (700 mL) and the filtrate was concentrated. The crude product thus obtained was dried under high vacuum and used without further purification.
Yield : 124 g (quantitative)
TLC : Rf= 0.38 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR (400 MHz, OMSO-d6) δ 7.31 (br s, IH), 6.86 (d, J = 12.0 Hz, 2H), 6.48 (d, J = 12.0 Hz, 2H), 4.60 (s, 2H), 3.71 (br s, 2H), 3.38 (br s, 2H), 1.44 (s, 9H)
LCMS: 277 (M+H+) (C14H20N4O2)
Step 6: Preparation of N-(3-(5,6-dihydro-4-t-butoxycarbonyl-[l,2,4]triazin-l-yl)anilino-(2R)-2-hydroxypropyl)-5-chloro-2-thiophene carboxamide

The compound obtained in Step 5 (120 g, 435 mmol) and 5-chloro-N-(((S)-oxiran-2-yl)methyl)thiophene-2-carboxamide (123 g, 566 mmol, 1.3 eq, purchased from RStech (Daejeon, Korea) was suspended in absolute EtOH (1450 mL). The mixture thus obtained was stirred at 85 °C for 16 hrs. The mixture was cooled to r.t. and evaporated in vacuo to remove solvent. The resultant residue was dried under high vacuum for 18 hrs. The dried solid was suspended in EA (2 L). The suspension thus obtained was stirred at r.t. for 1 hr. The solid thus obtained was filtered off and washed with EA (500 mL) and ether (500 mL). The filtered solid was dried under high vacuum to obtain the title compound.
Aniline (starting material), epoxide, over-reacted by product were contained in crude product.
Yield : 158 g (74 %)
TLC : Rf= 0.34 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR of a crude sample (400 MHz, DMSO-^6) δ 8.57 (t, J = 5.4 Hz,
IH), 7.65 (d, J = 3.6 Hz, IH), 7.32 (br s, IH), 7.14 (d, J = 4.2 Hz, IH), 6.90 (d, J
= 9.0 Hz, 2H), 6.51 (d, J = 9.0 Hz, 2H), 5.04 (t, J = 6.6 Hz, IH), 5.00 (d, J = 5.4 Hz, IH), 3.87-3.65 (m, 3H), 3.40 (br s, 2H), 3.37-3.34 (m, IH), 3.25-3.21 (m, IH),
3.17-2.96 (m, IH), 2.94-2.84 (m, IH), 1.44 (s, 9H)
LCMS: 494 (M+H+) (C22H28ClN5O4S)
Step 7: Preparation of 5-chloro-N-({(5S)-2-oxo-3-[(5,6-dihydro-4-t-butoxycarbonyl-[l,2,4]triazin-l-yl)phenyl]-l,3-oxazolidin-5-yl}-methyl)-2-thiophene carboxamide

The compound obtained in Step 6 (158 g, 320 mmol) was suspended in
THF (1000 niL), and 1,1-carbonyldiimidazole (68 g, 416 mmol, 1.3 eq, Aldrich 115533) and DMAP (2 g, 16 mmol, 0.05 eq, Aldrich 107700) were added thereto. The mixture thus obtained was stirred at 75 °C for 3 hrs, cooled to r.t, and evaporated in vacuo to remove solvent. The resultant residue was suspended in EtOH (1300 mL). The suspension thus obtained was stirred at 0 °C for 1 hr. The solid thus produced was filtered off and washed with cold EtOH (800 mL) and cold MeOH (300 mL). The filtered solid was dried under high vacuum to obtain the title compound.
Yield : 101 g (61 %)
TLC : R/= 0.75 (EA/MeOH/AcOH = 20/1/0.5)
1H NMR (400 MHz, DMSO-^6) δ 8.93 (t, J= 5.4 Hz, IH), 7.66 (d, J= 4.2 Hz, IH), 7.43-7.33 (m, 3H),7.29-7.12 (m, 3H), 4.82-4.73 (m, IH), 4.09 (t, J = 9.0 Hz, IH), 3.82-3.70 (m, 3H), 3.65-3.52 (m, 4H), 1.45 (s, 9H)
LCMS: 520 (M+H+) (C23H26ClN5O5S)
Step 8: Preparation of 5-chloro-N-({(5S)-2-oxo-3-[(5,6-dihydro-4H-[l,2,4]triazin-l-yl)phenyl]-l,3-oxazolidin-5-yl}-methyl)-2-thiophene
carboxamide hydrochloride

The compound obtained in Step 7 (101 g, 194 mmol) was suspended in aq.
3N HCl solution (1.1 L) and THF (1.1 L), and stirred at 80 “C for 3 hrs. The mixture thus obtained was cooled to r.t. The solid thus produced was filtered off, washed with THF (700 mL) and dried under high vacuum to obtain the title compound.
Yield : 75 g (85 %)
TLC : Rf= 0.82 (MC/MeOH/AcOH = 10/1/0.5)
1H NMR (400 MHz, DMSO-J6) δ 12.12 (br s, IH), 10.32 (br s, IH), 9.13
(t, J = 6.0 Hz, IH), 8.57 (d, J= 5.2 Hz, IH), 7.75 (d, J = 4.2 Hz, IH), 7.49 (d, J =
9.0 Hz, 2H), 7.15 (d, J= 4.2 Hz, IH), 7.09 (d, J= 9.0 Hz, 2H), 4.85-4.74 (m, IH), 4.11 (t, J = 8.8 Hz, IH), 3.85 (dd, J = 6.0, 9.2 Hz, IH), 3.62 (t, J = 4.8 Hz, 2H),
3.59-3.49 (m, 2H), 3.15 (br s,2H)
LCMS: 420 (M+H+) (C18H18ClN5O3)
Example 5: Preparation of 5-chloro-N-({(5S)-2-oxo-3-[(5,6-dihydro-4H-[l,2,4]triazin-l^yl)phenyl]-l,3-oxazolidin-5-yl}-methyl)-2-thiophene
carboxamide

The compound obtained in Example 4 (20 g, 43.8 mmol) was suspended in MeOH/H2O (1/2 wt/wt, 3.2 L) and stirred at 100 °C until the compound obtained in Example 4 was dissolved clearly. 0.6M aq. Na2CO3 solution (75 mL) was added thereto. The mixture thus obtained was stood at 0 °C for 2 hrs. The solid thus produced was filtered off, washed with H2O (400 mL) and dried
under high vacuum to obtain the title compound.
Yield : 17 g (93 %)
1H NMR (400 MHz, DMSO-J6) δ 8.93 (t, J = 6.0 Hz, IH), 7.66 (d, J = 4.0 Hz, IH), 7.29 (d, J = 9.2 Hz, 2H), 7.16 (d, J = 4.0 Hz, IH), 7.08 (d, J = 9.2 Hz, 2H), 6.76 (d, J = 4.0 Hz, IH), 6.48 (br s, IH), 4.78-4.69 (m, IH), 4.07 (t, J = 8.8 Hz, IH), 3.74 (dd, J = 6.0, 9.2 Hz, IH), 3.54 (t, J = 5.6 Hz, 2H), 3.38 (s, 4H)
LCMS: 420 (M+H+) (C18H18ClN5O3)
Example 6: Preparation of 5-chIoro-N-({(5S)-2-oxo-3-[(5,6-dihydro-4H-[l,2,4]triazin-l-yl)phenyI]-l,3-oxazolidin-5-yl}-methyl)-2-thiophene
carboxamide methane sulfonate

The compound obtained in Example 5 (16.7 g, 39.8 mmol) was suspended in MeOH/CH2Cl2 (1/4 v/v, 350 mL) and stirred at 50 °C until the compound obtained in Example 5 was dissolved clearly. The mixture thus obtained was cooled to 0 °C and methylsulfonic acid (2.9 mL, 43.8 mmol, 1.3 eq, Aldrich 471356) was added thereto at 0 °C . The resulting mixture was evaporated in vacuo to remove solvent. The resultant solid was suspended in absolute EtOH (100 mL) and the suspension was stirred at 90 °C to dissolve solid clearly. The resulting mixture was cooled to 0 °C and stirred at 0 °C for 2 hrs. The solid thus produced was filtered off, washed with absolute EtOH (100 mL), and dried under high vacuum to obtain the title compound.
Yield : 18.4 g (89.7 %)
1H NMR (400 MHz, DMSO-J6) δ 11.93 (br s, IH), 10.03 (br s, IH), 8.94 (t, J = 6.0 Hz, IH), 8.55 (d, J = 6.0 Hz, IH), 7.66 (d, J = 4.0 Hz, IH), 7.49 (d, J = 9.2 Hz, 2H), 7.16 (d, J = 4.0 Hz, IH), 7.08 (d, J = 9.2 Hz, 2H), 4.93-4.87 (m, IH), 4.10 (t, J = 9.2 Hz, IH), 3.77 (dd, J = 6.0, 9.2 Hz, IH), 3.63 (m, 2H), 3.57 (t, J = 5.6 Hz, 2H), 3.16 (br s, 2H), 2.28 (s, 3H)
LCMS: 420 (M+H+) (C18H18ClN5O3)
PATENT
WO2010002115
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2010002115
[Reaction Scheme 1] [96] A., O
NCONH2 + &J\ – NC NC- boc IPA, reflux O*B£.H. .κ> boc DMAP boc 2

Example 10: Preparation of compound 109

Compound 15a (450 mg, 0.88 mmol) obtained in Manufacturing Example 3 was dissolved in dichloromethane (10 mL), to which HCl (4 M 1,4-dioxane solution) (10 mL) was added, followed by stirring at room temperature for 1 hour. The reactant was concentrated under reduced pressure and dried to give light yellow solid compound (425 mg, 0.88 mmol, 100%). This compound (392 mg, 0.81 mmol) was dissolved in acetic acid (4 mL), to which trimethylorthoformate (2 mL) was added, followed by reflux with stirring. 10 hours later, after solvent was evaporated all, column chromatography (dichlorome thane/me thanol(v/v) 20/1 → 12/1) was performed to give the title compound 109 as a light yellow solid (215 mg, 5.12 mmol, 63 %).
1H NMR (400 MHz, CDCl3) δ 7.35 (d, J = 9.2 Hz, 2H), 7.33 (d, J = 4.4 Hz, IH), 7.14 (d, J = 9.2 Hz, 2H), 7.01 (t, J = 6.4 Hz, IH), 6.88 (s, IH), 6.85 (d, J = 4.4 Hz, IH), 4.87-4.79 (m, IH), 4.06 (t, J = 9 Hz, IH), 3.86 (ddd, J = 14.4 ,6, 3 Hz, IH), 3.81 (dd, J = 9, 6.4 Hz, IH), 3.69 (dt, J = 14.4, 6 Hz, IH), 3.62-3.58 (m, 2H), 3.55-3.51 (m, 2H); LCMS: 420 (M+H+) to Ci8H18ClN5O3S
REFERENCES
https://clinicaltrials.gov/ct2/show/NCT01954238
SEE EARLIER MOLECULE LCB01-0371…..http://newdrugapprovals.org/2014/03/31/lcb01-0371-new-oxazolidinone-has-improved-activity-against-gram-positive-pathogens/
////////////////phase 1, Green Cross Corp, LegoChem Bioscience, GCC 4401C, thrombosis, venous thromboembolism, GC 2107, CB02-0133, GC-2107, GC4401, GCC-2107, GCC-4401, GCC-4401C, I Fxa – LegoChem Biosciences, LCB02-0133, Nokxaban
O=C(NC[C@H]3CN(c1ccc(cc1)N2CCNC=N2)C(=O)O3)c4ccc(Cl)s4.CS(=O)(=O)O METHANE SULFONATE
O=C(NC[C@H]3CN(c1ccc(cc1)N2CCNC=N2)C(=O)O3)c4ccc(Cl)s4 FREE FORM
C1CN(NC=N1)C2=CC=C(C=C2)N3CC(OC3=O)CNC(=O)C4=CC=C(S4)Cl