Cipargamin, NITD 609
IUPAC Name: (3R,3'S)-5,7'-dichloro-6'-fluoro-3'-methylspiro[1H-indole-3,1'-2,3,4,9-tetrahydropyrido[3,4-b]indole]-2-one |
CAS Registry Number: 1193314-23-6
Synonyms: NITD609, NITD 609, NITD-609, GNF-609
KAE-609
NITD-609
Synonyms: NITD609, NITD 609, NITD-609, GNF-609
KAE-609
NITD-609
390.238, C19 H14 Cl2 F N3 O
(1'R,3'S)-5,7'-Dichloro-6'-fluoro-3'-methyl-1,2,2',3',4',9'-hexahydrospiro[indole-3,1'-pyrido[3,4-b]indole]-2-one
(1R,3S)-5′,7-Dichloro-6-fluoro-3-methyl-2,3,4,9-tetrahydrospiro[β-carboline-1,3′-indol]-2′(1′H)-one
CURRENTLY IN -PHASE2
NITD609 is an experimental synthetic antimalarial molecule belonging to the spiroindolone class.[1][2] The compound was developed at the Novartis Institute for Tropical Diseases in Singapore, through a collaboration with the Genomics Institute of the Novartis Research Foundation (GNF), the Biomedical Primate Research Centre and the Swiss Tropical Institute.
NITD609 is a novel, synthetic antimalarial molecule belonging to the
spiroindolone class, awarded MMV Project of the Year 2009.
It is structurally related to GNF 493, a compound first identified as a potent inhibitor of Plasmodium falciparum growth in a high throughput phenotypic
screen of natural products conducted at the Genomics Institute of the
Novartis Research Foundation in San Diego, California in 2006. NITD609
was discovered by screening the Novartis library of 12,000 natural
products and synthetic compounds to find compounds active against Plasmodium falciparum. The first screen turned up 275 compounds and the list was narrowed to 17 potential candidates.
KAE609
(cipargamin; formerly NITD609, Novartis Institute for Tropical
Diseases) is a new synthetic antimalarial spiroindolone analogue with
potent, dose-dependent antimalarial activity against asexual and sexual
stages of Plasmodium falciparum.http://www.nejm.org/doi/full/10.1056/NEJMoa1315860
KAE609 shows promise as next generation treatment for malaria
http://www.novartis.com/newsroom/media-releases/en/2014/1843976.shtml- KAE609
is the first antimalarial drug candidate with a novel mechanism of
action to achieve positive clinical proof-of-concept in over 20 years
- KAE609
was tested in adult patients with uncomplicated malaria and showed a
median parasite clearance time of 12 hours, including in patients with
resistant infections[1]
- For more than a decade, Novartis has been a leader in the fight against malaria, setting the current gold standard for treatment and building one of the strongest malaria pipelines in the industry

KAE609 shows promise as next generation treatment for malaria
- KAE609
is the first antimalarial drug candidate with a novel mechanism of
action to achieve positive clinical proof-of-concept in over 20 years
- KAE609
was tested in adult patients with uncomplicated malaria and showed a
median parasite clearance time of 12 hours, including in patients with
resistant infections[1]
- For more than a decade, Novartis has been a leader in the fight against malaria, setting the current gold standard for treatment and building one of the strongest malaria pipelines in the industry
Basel, Switzerland, July 30, 2014 - Today, Novartis published clinical trial results in the New England Journal of Medicine showing that KAE609 (cipargamin), a novel and potent antimalarial drug candidate, cleared the parasite rapidly in Plasmodium falciparum (P. falciparum) and Plasmodium vivax (P. vivax)
uncomplicated malaria patients[1]. Novartis currently has two drug
candidates in development. Both KAE609 and KAF156 are new classes of
anti-malarial compounds that treat malaria in different ways from
current therapies, important to combat emerging drug resistance.
Novartis has also identified PI4K as a new drug target with potential to
prevent, block and treat malaria.
"Novartis is in
the fight against malaria for the long term and we are committed to the
continued research and development of new therapies to eventually
eliminate the disease," said Joseph Jimenez, CEO of Novartis. "With two
compounds and a new drug target currently under investigation, Novartis
has one of the strongest malaria pipelines in the industry."
Malaria is a life-threatening disease primarily caused by parasites (P. falciparum and P. vivax)
transmitted to people through the bites of infected Anopheles
mosquitoes. Each year it kills more than 600,000 people, most of them
African children[2].
"KAE609 is a potential
game-changing therapy in the fight against malaria," said Thierry
Diagana, Head of the Novartis Institute for Tropical Diseases (NITD),
which aims to discover novel treatments and prevention methods for major
tropical diseases. "Novartis has given KAE609 priority project status
because of its unique potential of administering it as a single-dose
combination therapy."
In June 2012, 21 patients
infected by one of the two main malaria-causing parasite types took part
in a proof-of-concept clinical study conducted in Bangkok and Mae Sot
near the Thailand/Burma border where resistance to current therapies had
been reported. Researchers saw rapid parasite clearance in adult
patients (median of 12 hours)[2] with uncomplicated P. vivax or P. falciparum
malaria infection including those with resistant parasites. No safety
concerns were identified, however the study was too small for any safety
conclusions.
"The growing menace of artemisinin
resistance threatens our current antimalarial treatments, and therefore
our attempts to control and eliminate falciparum malaria," said Nick
White, Professor of Tropical Medicine at Mahidol University in Thailand
and lead author of the NEJM article. "This is why we are so enthusiastic
about KAE609; it is the first new antimalarial drug candidate with a
completely novel mechanism of action to reach Phase 2 clinical
development in over 20 years."
KAE609, the first
compound in the spiroindolone class of treatment, works through a novel
mechanism of action that involves inhibition of a P-type
cation-transporter ATPase4 (PfATP4), which regulates sodium
concentration in the parasite. Because KAE609 also appears to be
effective against the sexual forms of the parasite, it could potentially
help prevent disease transmission. The clinical trial was done in
collaboration with the Wellcome Trust-Mahidol University - Oxford
Tropical Medicine Research Programme. Research was supported by the
Wellcome Trust, Singapore Economic Development Board, and Medicines for
Malaria Venture.
KAE609 represents one of two new
classes of antimalarial compounds that Novartis has discovered and
published in the last four years.[3],[4] This drug candidate has shown
potent in vitro activity against a broad range of parasites that have
developed drug resistance against current therapies. KAE609 is currently
being planned for Phase 2b trials.
References
[1] http://www.nejm.org/doi/full/10.1056/NEJMoa1315860
[2] World Health Organization, http://www.who.int/mediacentre/factsheets/fs094/en/
[3] Spiroindolones, a Potent Compound Class for the Treatment of Malaria, KAE609, Science, Sept. 2010
[4] Imaging of Plasmodium liver stages to drive next generation antimalarial drug discovery. Science Express, Nov. 17, 2011
[1] http://www.nejm.org/doi/full/10.1056/NEJMoa1315860
[2] World Health Organization, http://www.who.int/mediacentre/factsheets/fs094/en/
[3] Spiroindolones, a Potent Compound Class for the Treatment of Malaria, KAE609, Science, Sept. 2010
[4] Imaging of Plasmodium liver stages to drive next generation antimalarial drug discovery. Science Express, Nov. 17, 2011

The current spiroindolone
was optimized to address its metabolic liabilities leading to improved
stability and exposure levels in animals. As a result, NITD609 is one of
only a handful of molecules capable of completely curing mice infected
withPlasmodium berghei (a model of blood-stage malaria).
Given
its good physicochemical properties, promising pharmacokinetic and
efficacy profile, the molecule was recently approved as a preclinical
candidate and is now entering GLP toxicology studies with the aim of
entering Phase I studies in humans in late 2010. If its safety and
tolerability are acceptable, NITD609 would be the first antimalarial not
belonging to either the artemisinin or peroxide class to go into a proof-of-concept study in malaria.
If
NITD609 behaves similarly in people to the way it works in mice, it may
be possible to develop it into a drug that could be taken just once -
far easier than current standard treatments in which malaria drugs are
taken between one and four times a day for up to seven days. NITD609
also has properties which could enable it to be manufactured in pill
form and in large quantities. Further animal studies have been performed
and researchers have begun human-stage trials.
 | |
| Identifiers | |
| ChemSpider | 24662493 |
| Jmol-3D images | Image 1 |
| Properties | |
| Molecular formula | C19H14Cl2FN3O |
| Molar mass | 390.24 g mol−1 |
Leishmaniasis is caused by one of more than twenty (20) varieties of parasitic protozoa that belong to the genus Leishmania, and is transmitted by the bite of female sandflies. Leishmaniasis is endemic in some 90 countries, including many tropical and sub-tropical areas.
There are four main forms of leishmaniasis. Visceral leishmaniasis, also called kala-azar, is the most serious form and is caused by the parasite Leishmania donovani. Patients who develop visceral leishmaniasis can die within months unless they receive treatment. The two main therapies for visceral leishmaniasis are the antimony derivatives sodium stibogluconate (Pentostam®) and meglumine antimoniate (Glucantim®). Sodium stibogluconate has been used for about 70 years and resistance to this drug is a growing problem. In addition, the treatment is relatively long and painful, and can cause undesirable side effects. Human African Trypanosomiasis, also known as sleeping sickness, is a vector-bome parasitic disease. The parasites concerned are protozoa belonging to the Trypanosoma Genus. They are transmitted to humans by tsetse fly {Glossina Genus) bites which have acquired their infection from human beings or from animals harbouring the human pathogenic parasites.
Chagas disease (also called American trypanosomiasis) is another human parasitic disease that is endemic amongst poor populations on the American continent. The disease is caused by the protozoan parasite Trypanosoma cruzi, which is transmitted to humans by blood-sucking insects. The human disease occurs in two stages: the acute stage, which occurs shortly after the infection, and the chronic stage, which can develop over many years. Chronic infections result in various neurological disorders, including dementia, damage to the heart muscle and sometimes dilation of the digestive tract, as well as weight loss. Untreated, the chronic disease is often fatal.
The drugs currently available for treating Chagas disease are nifurtimox and benznidazole. However, problems with these current therapies include their adverse side effects, the length of treatment, and the requirement for medical supervision during treatment. Furthermore, treatment is really only effective when given during the acute stage of the disease. Resistance to the two frontline drugs has already arisen. The antifungal agent amphotericin b has been proposed as a second-line drug, but this drug is costly and relatively toxic.
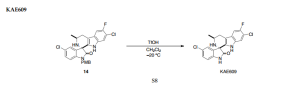
PAPER
Stereoselective Total Synthesis of KAE609 via Direct Catalytic Asymmetric Alkynylation to Ketimine
† Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021, Japan
‡ JST, ACT-C, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo 141-0021, Japan
Org. Lett., 2015, 17 (19), pp 4762–4765
DOI: 10.1021/acs.orglett.5b02300
Publication Date (Web): September 14, 2015
Copyright © 2015 American Chemical Society
*E-mail: nkumagai@bikaken.or.jp., *E-mail: mshibasa@bikaken.or.jp.
Abstract

A
direct catalytic asymmetric alkynylation protocol is applied to provide
the requisite enantioenriched propargylic α-tertiary amine, allowing
for the stereoselective total synthesis of KAE609 (formerly NITD609 or
cipargamin).

CLICK ON IMAGE TO VIEW
http://pubs.acs.org/doi/abs/10.1021/acs.orglett.5b02300?journalCode=orlef7
http://pubs.acs.org/doi/suppl/10.1021/acs.orglett.5b02300/suppl_file/ol5b02300_si_001.pdf
PATENT
WO 2009/132921

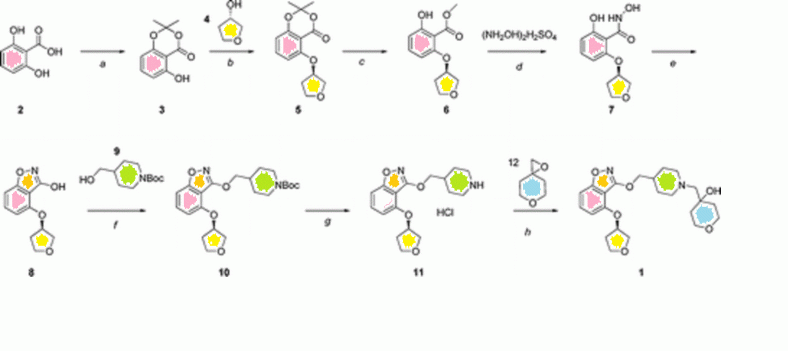
In this process, the chiral amine is installed via an enzymatic resolution via deacylation of the acetamide 2. In addition to the wasteful resolution, other inefficiencies of this route include protection/deprotection (Ac/Boc, 2 to 4, and 5 to 6) and a three-step sequence to reduce the carboxylic acid to a methyl group (3 to 6).
Patent
US 2015/0045562

Improved Route to Cipargamin Employing Transaminase Reaction
For the transamination step, the enzyme ATA-256 was engineered by Codexis to accommodate the non-natural indole substrate 12.
Since the substrate is not water-soluble, PEG 200 (approximately 20 vol
%) is used as a cosolvent, an interesting selection given that DMSO or
methanol are the most common cosolvents for enzymatic reactions.
Isopropylamine is employed as the amine donor, a strategy that was
adopted from the work of Merck and Codexis for the transamination of
sitagliptin ketone.(2) During the transamination, which is a reversible reaction,i-PrNH2
is converted to acetone, which can be readily removed by evaporation to
drive the reaction to completion. The workup involves filtration to
remove enzyme residues followed by pH swings in which the product is
extracted into the aqueous layer under acidic conditions, then basified
for extraction into the organic layer. Addition of (+)-camphorsulfonic
acid (CSA) provides the amine 14 as the crystalline CSA salt. No
details are provided on enantioselectivity for the transamination, and
it is not clear if the (+)-CSA is required to upgrade the ee or whether
this salt was selected based on physical properties and the ability to
develop a scalable crystallization process.
The final step to generate the spiroindole involves a diastereoselective condensation of the chiral amine with 5-chloroisatin (7)
under acidic conditions. The diastereoselectivity of this reaction is
not provided, nor any ee or de data for the final product. The
spiroindole is also isolated as a (+)-CSA salt, which is then converted
to the crystalline free base hemihydrate as the final form of
cipargamin.
622.54 399.25
In a 750ml reactor with impeller stirrer 50g of compound (IVB) salt were dissolved in 300ml Ethanol (ALABD) and 100 ml deionised Water (WEM). The clear, yellowish sollution was heated to 58°C internal temperature. To the solution 85 g of a 10% aqueous sodium carbonate solution was added within 10 minutes. The clear solution was particle filtered into a second reaction vessel. Vessel and particle filter were each rinsed with 25 ml of a mixture of ethanohwater (3:1 v/v) in the second reaction vessel. The combined particle filtered solution is heated to 58°C internal temperature and 200ml water (WEM) were added dropwise within 15 minutes. Towards the end of the addition the solution gets turbid. The mixture is stirred for 10 minutes at 58°C internal temperature and is then cooled slowely to room temperature within 4hours 30 minutes forming a thick, well stirable white suspension. To the suspension 200 ml water are added and the mixture is stirred for additional 15hours 20 minutes at room temperature. The suspension is filtered and the filter cake is washed twice with 25 ml portions of a mixture of ethanohwater 9: 1 (v/v). The colourless crystals are dried at 60°C in vacuum yielding 26.23g (=91.2% yield). H NMR (400 MHz, DMSO-d6)
0.70 (s, 1H), 10.52 (s, 1H), 7.44 (d, J = 10.0 Hz, 1H), 7.33 (dd, J = 8.4, 2.1 Hz, 1H),.26 (d, J = 6.5 Hz, 1H), 7.05 (d, J = 2.3 Hz, 1H), 6.93 (d, J = 8.3 Hz, 1H), 3.83 - 4.00 (m,H), 3.13 (d, J = 6.0 Hz, 1H), 2.77 (dd, J = 15.1, 3.8 Hz, 1H), 2.38 (dd, J = 15.1, 10.5 Hz,H), 1.17 (d, J = 6.3 Hz, 3H).
Patent

 http://www.google.com/patents/WO2009132921A1?cl=en
http://www.google.com/patents/WO2009132921A1?cl=en
SCHEME G: Preparation of (lR,3S)-5',7-dichloro-6-fluoro-3-methyl-2,3,4,9- tetrahydrospiro[β-carboline-l,3'-indol-2'(l'iϊ)-one (35) and (lR,3S)-5'-chloro-6-fluoro-3- methyl-2,3,4,9-tetrahydrospiro[β-carboline-l,3'-indoI-2'(l'H0-one (36)
Step 1 : POCl3 (2.43 mL, 26.53 mmol) was added dropwise to N, N-dimethylformamide (15.0 mL) at -20 °C and stirred below -5 0C for one hour. A solution of 6-chloro-5-fluoroindole (3.0 g, 17.69 mmol) in dimethylformamide (5.0 mL) was added dropwise to the above reaction mixture at -20 °C. The salt-ice bath was removed and the reaction mixture was warmed to 35 0C, After one hour, the reaction was poured onto ice and basified by solid sodium bicarbonate and extracted with ethyl acetate. The combined organic layer was washed with water and then concentrated to give 6-chloro-5-fluoro-1H-indole-3-carbaldehyde (3.4 g, 97 %) as a light brown solid. 1H ΝMR (500 MHz, CDCl3): δ 10.02 (s, 1 H), 8.10 (d, IH, J = 9.5 Hz), 7.87 (s, 1 H), 7.49 (d, IH, J= 5.5 Hz).
Step 2: The solution (0.2 M) of 6-chloro-5-fluoro-1H-indole-3-carbaldehyde (4.0 g, 20.24 mmol) in nitroethane (100 mL) was refluxed with ammonium acetate (1.32 g, 0.85 mmol) for 4 hours. The reaction mixture was concentrated under vacuum to remove nitroethane, diluted with ethylacetate and washed with brine. The organic layer was concentrated to give 6-chloro-5- fluoro-3-(2-nitro-propenyl)-1H-indole (5.0 g, 97 %) as a reddish orange solid. 1H ΝMR (500 MHz, CDCl3): δ 8.77 (s, IH), 8.32 (s, IH), 7.58 (d, IH, J= 2.5 Hz), 7.54 (d, IH, J = 9 Hz), 7.50 (d, IH, J= 5.9 Hz), 2.52 (s, 3H). Step 3: A solution of 6-chloro-5-fluoro-3-(2-nitro-propenyl)-1H-indole (5.0 g, 19.63 mmol) in tetrahydrofuran (10 mL) was added to the suspension of lithium aluminium hydride (2.92 g, 78.54 mmol) in tetrahydrofuran (20 mL) at 0 0C and then refluxed for 3 hours. The reaction mixture was cooled to 0 °C, and quenched according to the Fischer method. The reaction mixture was filtered through celite and the filtrate concentrated to give 2-(6-chloro-5-fluoro-1H-indol-3- yl-1-methyl-ethylamine (4.7 g crude) as a viscous brown liquid. The residue was used without further purification. 1H NMR (500 MHz, CDCl3): δ 8.13 (s, IH), 7.37 (d, IH, 6.Hz), 7.32 (d, IH, J = 10 Hz), 7.08 (s, IH), 3.23-3.26 (m, IH), 2.77-2.81 (m, IH), 2.58-2.63 (m, IH), 1.15 (d, 3H, J= 6.5 Hz).
Step 4: A mixture of 2-(6-chloro-5-fluoro-1H-indol-3-yl-l-methyl-ethylamine (4.7 g, 20.73 mmol), 5-chloroisatin (3.76 g, 20.73 mmol) and p-toluenesulphonic acid (394 mg, 2.07 mmol) in ethanol (75 mL) was refluxed overnight. The reaction mixture was concentrated to remove ethanol, diluted with ethyl acetate and washed with saturated aqueous NaHCO3. The organic layer was concentrated to give a brown residue, which was purified by silica gel chromatography (20 % ethyl acetate in hexane) to provide the corresponding racemate (4.5 g, 56 %) as a light yellow solid. The racemate was separated into its enantiomers by chiral chromatography to provide 35.
Compound 36 can be obtained in a similar fashion from 5-fluoroindole.
Alternatively 35 and 36 were be prepared in enantiomerically pure form by the following scheme.
SCHEME H: Alternative preparation of (lR,3S)-5',7-dichloro-6-fluoro-3-methyl-2,3,4,9- tetrahydrospiro[β-carboline-l,3'-indol-2'(1'H)-one (35)
Step 1 : To a solution of 6-chloro-5-fluoroindole (1.8 g, 10.8 mmol) and Ac2O (10 niL) in AcOH (3OmL) was added L-serine (2.2 g, 20.9 mmol), the mixture was heated to 80 °C. After TLC indicated the reaction was complete, the mixture was cooled to 0 °C, neutralized to pH 11 , and washed with MTBE. The aqueous phase was acidified to pH 2 and extracted with EtOAc. The combined organic layers were washed with water and bπne, dπed with Na2SO4, filtered, and concentrated. The residue was purified with chromatography (Petroleum ether /EtOAc 1:1) to give 2-acetylamino-3-(6-chloro-5-fluoro-1H-mdol-3-yl)-propπonic acid as a light yellow solid (1.2 g, 37% yield).
Step 2: 2-Acetylamino-3-(6-chloro-5-fluoro-1H-indol-3-yl)-proprionic acid (2.5g, 8.4mmol) was dissolved in aqueous NaOH (IN, 10 niL) and water added (70 mL). The mixture was heated to 37-380C and neutralized with HCl (IN) to pΗ 7.3-7.8. L-Aminoacylase (0.5 g) was added to the mixture and allowed to stir for 2 days, maintaining 37-380C and pΗ 7.3-7.8. The mixture was heated to 60 °C for another hour, concentrated to remove part of water, cooled and filtered. The filtrate was adjusted to pΗ 5.89 and filtered again. The filtrate was adjusted to pΗ 2.0 and extracted with EtOAc. The combined organic layer was dried over Na2SO4, filtered, concentrated and the residue was purified with chromatography (petroleum ether /EtOAc 1 : IEtOAc) to give R- 2-acetylamino-3-(6-chloro-5-fluoro-1H-mdol-3-yl)-propπonic acid as a light yellow solid (1.2 g, 48% yield). Step 3: R-2-acetylamino-3-(6-chloro-5-fluoro-1H-indol-3-yl)-proprionic acid (1.2 g, 4.0 mmol) was dissolved in HCl (6N, 10 mL) and the mixture heated to reflux for 4 hours, and then concentrated to dryness. Toluene (50 mL) was added to the residue and concentrated to dryness to remove water and HCl. The residue was dried under vacuum and then dissolved in MeOH (20 mL). To the solution was added dropwise SOCl2 (0.5 mL, 6.8 mmol) at 0 °C, and the mixture was stirred overnight. After removal of solvent, the residue was dissolved in THF/water (40/10 mL) and NaHCO3 (1.0 g, 11.9 mmol) was added portionwise. Upon basifϊcation, BoC2O (1.2 g, 5.5 mmol) added at 0 °C and allowed to stir at room temperature. After TLC indicated the reaction was finished, EtOAc was added and separated and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with water and brine, dried with Na2SO4, filtered, concentrated and the residue was purified with chromatography (petroleum ether /EtOAc: 5/1) to give R-2-tert-butoxycarbonylamino-3-(6-chloro-5-fluoro-l/-/-indol-3-yl)-proprionic acid methyl ester 460 g, 31% yield for 3 steps).
Step 4: To a solution of R-2-tert-butoxycarbonylamino-3-(6-chloro-5-fluoro-l//-indol-3-yl)- proprionic acid methyl ester (460mg, 1.2mmol) in dry ether (20 mL) was added portionwise LiAlH4 (92 mg, 2.4 mmol) at 0 °C. The mixture was heated to reflux for 2 hours. After TLC indicated the reaction was finished, the mixture was cooled and carefully quenched with Na2SO4. The mixture was filtered and the filtrate was washed with saturated aqueous NH4Cl and water, dried with Na2SO4, filtered, concentrated to give a crude product (400 mg), which was used without further purification.
Step 5: To a solution of the crude product (400 mg, 1.2mmol) and Et3N (0.3 mL, 2.2 mmol) in CH2Cl2 (5 mL) was added MsCl (160 mg, 1.4 mmol) dropwise at 0 °C. The mixture was stirred for 2 hours at room temperature. After TLC indicated the reaction was completed, the mixture was washed with water and brine, dried with Na2SO4, filtered, concentrated and the residue was purified with chromatography (petroleum ether/EtOAc 5:1) to give methansulfonic acid (R)-2- ?ert-butoxycarbonylamino-3-(6-chloro-5-fluoro-1H-indol-3-yl)-propyl ester as a light yellow solid (300 mg, 57% yield, 2 steps)
Step 6: To a solution of mesylate (300 mg, 0.7mmol) in dry ether (20 mL) was added portionwise LiAlH4 (55 mg, 1.4 mmol) at 0 °C. The mixture was stirred at room temperature overnight. After TLC indicated the reaction was finished, the mixture was cooled and carefully quenched with Na2SO4. The mixture was filtered and the filtrate was washed with saturated aqueous NH4Cl and water, dried with Na2SO4, filtered, concentrated and the residue was purified with chromatography (petroleum ether/EtOAc 10: 1) to give [(5)-2-(6-chloro-5-fluoro-1H-indol-3-yl)- 1 -methyl-ethyl] -carbamic acid tert-butyl ester as a light yellow solid (200 mg, 87% yield).
Step 7: A solution of [(S)-2-(6-chloro-5-fluoro-1H-indol-3-yl)-l-methyl-ethyl]-carbamic acid tert-butyl ester (200 mg, 0.6 mmol) in HCl/MeOH (10 mL) was stirred at room temperature. After TLC indicated the reaction was finished, the mixture was concentrated to remove the solvent. To the residue was added EtOAc (5OmL), and the mixture was neutralized with saturated NaHCO3 to pH 8~9, and then extracted with EtOAc. The combined organic phases were dried with Na2SO4, filtered, concentrated to give a crude (S)-2-(6-chloro-5-fluoro-1H-indol-3-yl)-l- methyl-ethylamine which was used without further purification.
Step 8: To a solution of (5)-2-(6-chloro-5-fluoro-1H-indol-3-yl)-l-methyl-ethylamine (120 mg, 0.5 mmol) in EtOH (1OmL) was added 5-chloroisatin (90 mg, 0.5 mmol) and p-TsOΗ (8 mg, 0.04 mmol). The mixture was heated in a sealed tube at 1100C for 16 hours. After TLC indicated the reaction was finished, the mixture was cooled and concentrated. The residue was dissolved in EtOAc (2OmL) and washed with NaOH (IN) and brine, dried with Na2SO4, filtered, concentrated and the residue was purified with chromatography (petroleum ether/EtOAc 5:1) to give 36 (150mg, 64% yield over two steps).
Example 48 (15,3R)-5'-Chloro-3-methyl-2,3,4,9-tetrahydrospiro[β-carboline-l,3'-indol]-2'(l'JH)-one
(35)
35
Compound 35 may be prepared according to Scheme F using the same or analogous synthetic techniques and/or substituting with alternative reagents.
(lS^RVS'-Chloro-S-methyl-l^^^-tetrahydrospirotβ-carboline-l.S'-indoll-l^l'ZO-one: 1H NMR (300 MHz, DMSO-^6): δ 10.45 (s, IH), 10.42 (s, IH), 7.43 (d, J= 7.5 Hz, IH), 7.31 (dd, J = 2.1, 8.4 Hz, IH), 7.16 (d, J = 7.2 Hz, IH), 7.05-7.02 (m, 2H), 7.00-6.96 (m, IH), 6.92 (d, J = 8.1 Hz, IH), 3.98-3.86 (m, IH), 2.78 (dd, J= 3.6, 14.9 Hz, IH), 2.41 (dd, J= 4.5, 25.5 Hz, IH), 1.18 (d, J= 6.3 Hz, 3H); MS (ESI) m/z 338.0 (M+H)+.
Chiral compounds such as 36 and 37 can be prepared according to Scheme G or H using the same or analogous synthetic techniques and/or substituting with alternative reagents. Example 49
(IR^^-S'.T-Dichloro-ό-fluoro-S-methyl-l^^^-tetrahydrospiroIβ-carboline-l^'-indol]- 2\VH)-one (36)
36
35: 1H NMR (500 MHz, DMSO-Jd) δ 10.69 (s, IH), 10.51 (s, IH), 7.43 (d, J = 10.0 Hz, IH), 7.33 (dd, J= 8.4, 2.2 Hz, IH), 7.27 (d, J= 6.5 Hz, IH), 7.05 (d, J= 2.3, IH), 6.93 (d, J= 8.5 Hz, IH), 3.91 (m, IH), 3.13 (bd, J= 6.2 Hz, IH), 2.74 (dd, J= 15.0 , 3.0 Hz, IH), 2.35 (dd, J= 15.0, 10.3, IH), 1.15 (d, J= 6.0, 3H);
MS (ESI) m/z 392.0 (M+2H)+;
[α]25 D = + 255.4°
Example 50
(lS,3R)-5',7-Dichloro-6-fluoro-3-methyI-2,3,4,9-tetrahydrospiro[β-carboline-l,3'-indol]- 2'(l'H)-one (37)
37
(lS^^-S'^-Dichloro-o-fluoro-S-methyl^jS^^-tetrahydrospirojP-carboline-l-S'-indol]- 2'(l'H)-one: 1H NMR (500 MHz, CDCl3) δ 8.49 (s, IH), 7.54 (s, IH), 7.24 (d, J= 9.7 Hz, IH), 7.21 (dd, J = 8.6, 2.0 Hz, IH), 7.14 (d, J= 6.0 Hz, IH), 7.11 (d, J= 1.8, IH), 6.77 (d, J= 8.3 Hz, IH), 4.14 (m, IH), 2.89 (dd, J = 15.4, 3.7 Hz, IH), 2.49 (dd, J = 15.3, 10.5, IH), 1.68 (bs, IH), 1.29 (d, J= 6.4 Hz, 3H); MS (ESI) m/z 392.0 (M+2H)+; [α]25D -223.3°
PATENT
US 2011275613



 http://www.google.com/patents/WO2013139987A1?cl=en
http://www.google.com/patents/WO2013139987A1?cl=en
Prior art:
(1 'R, 3'S)-5, 7'-dichloro-6'-fIuoro-3'-methyl-2', 3',4', 9'-tetrahydrospiro[indoline-3, 1 - pyrido[3,4-b]indol]-2-one (eg. a compound of formula (IV), which comprises a spiroindolone moiety) and a 6-steps synthetic method for preparing, including known chiral amine intermediate compound (MA) are known (WO 2009/132921 ):
SCHEME G: Preparation of (lR,3S)-5',7-dichloro-6-fluoro-3-methyl-2,3,4,9- tetrahydrospiro[β-carboline-l,3'-indol-2'(l'iϊ)-one (35) and (lR,3S)-5'-chloro-6-fluoro-3- methyl-2,3,4,9-tetrahydrospiro[β-carboline-l,3'-indoI-2'(l'H0-one (36)
Step 1 : POCl3 (2.43 mL, 26.53 mmol) was added dropwise to N, N-dimethylformamide (15.0 mL) at -20 °C and stirred below -5 0C for one hour. A solution of 6-chloro-5-fluoroindole (3.0 g, 17.69 mmol) in dimethylformamide (5.0 mL) was added dropwise to the above reaction mixture at -20 °C. The salt-ice bath was removed and the reaction mixture was warmed to 35 0C, After one hour, the reaction was poured onto ice and basified by solid sodium bicarbonate and extracted with ethyl acetate. The combined organic layer was washed with water and then concentrated to give 6-chloro-5-fluoro-1H-indole-3-carbaldehyde (3.4 g, 97 %) as a light brown solid. 1H ΝMR (500 MHz, CDCl3): δ 10.02 (s, 1 H), 8.10 (d, IH, J = 9.5 Hz), 7.87 (s, 1 H), 7.49 (d, IH, J= 5.5 Hz).
Step 2: The solution (0.2 M) of 6-chloro-5-fluoro-1H-indole-3-carbaldehyde (4.0 g, 20.24 mmol) in nitroethane (100 mL) was refluxed with ammonium acetate (1.32 g, 0.85 mmol) for 4 hours. The reaction mixture was concentrated under vacuum to remove nitroethane, diluted with ethylacetate and washed with brine. The organic layer was concentrated to give 6-chloro-5- fluoro-3-(2-nitro-propenyl)-1H-indole (5.0 g, 97 %) as a reddish orange solid. 1H ΝMR (500 MHz, CDCl3): δ 8.77 (s, IH), 8.32 (s, IH), 7.58 (d, IH, J= 2.5 Hz), 7.54 (d, IH, J = 9 Hz), 7.50 (d, IH, J= 5.9 Hz), 2.52 (s, 3H). Step 3: A solution of 6-chloro-5-fluoro-3-(2-nitro-propenyl)-1H-indole (5.0 g, 19.63 mmol) in tetrahydrofuran (10 mL) was added to the suspension of lithium aluminium hydride (2.92 g, 78.54 mmol) in tetrahydrofuran (20 mL) at 0 0C and then refluxed for 3 hours. The reaction mixture was cooled to 0 °C, and quenched according to the Fischer method. The reaction mixture was filtered through celite and the filtrate concentrated to give 2-(6-chloro-5-fluoro-1H-indol-3- yl-1-methyl-ethylamine (4.7 g crude) as a viscous brown liquid. The residue was used without further purification. 1H NMR (500 MHz, CDCl3): δ 8.13 (s, IH), 7.37 (d, IH, 6.Hz), 7.32 (d, IH, J = 10 Hz), 7.08 (s, IH), 3.23-3.26 (m, IH), 2.77-2.81 (m, IH), 2.58-2.63 (m, IH), 1.15 (d, 3H, J= 6.5 Hz).
Step 4: A mixture of 2-(6-chloro-5-fluoro-1H-indol-3-yl-l-methyl-ethylamine (4.7 g, 20.73 mmol), 5-chloroisatin (3.76 g, 20.73 mmol) and p-toluenesulphonic acid (394 mg, 2.07 mmol) in ethanol (75 mL) was refluxed overnight. The reaction mixture was concentrated to remove ethanol, diluted with ethyl acetate and washed with saturated aqueous NaHCO3. The organic layer was concentrated to give a brown residue, which was purified by silica gel chromatography (20 % ethyl acetate in hexane) to provide the corresponding racemate (4.5 g, 56 %) as a light yellow solid. The racemate was separated into its enantiomers by chiral chromatography to provide 35.
Compound 36 can be obtained in a similar fashion from 5-fluoroindole.
Alternatively 35 and 36 were be prepared in enantiomerically pure form by the following scheme.
SCHEME H: Alternative preparation of (lR,3S)-5',7-dichloro-6-fluoro-3-methyl-2,3,4,9- tetrahydrospiro[β-carboline-l,3'-indol-2'(1'H)-one (35)
Step 1 : To a solution of 6-chloro-5-fluoroindole (1.8 g, 10.8 mmol) and Ac2O (10 niL) in AcOH (3OmL) was added L-serine (2.2 g, 20.9 mmol), the mixture was heated to 80 °C. After TLC indicated the reaction was complete, the mixture was cooled to 0 °C, neutralized to pH 11 , and washed with MTBE. The aqueous phase was acidified to pH 2 and extracted with EtOAc. The combined organic layers were washed with water and bπne, dπed with Na2SO4, filtered, and concentrated. The residue was purified with chromatography (Petroleum ether /EtOAc 1:1) to give 2-acetylamino-3-(6-chloro-5-fluoro-1H-mdol-3-yl)-propπonic acid as a light yellow solid (1.2 g, 37% yield).
Step 2: 2-Acetylamino-3-(6-chloro-5-fluoro-1H-indol-3-yl)-proprionic acid (2.5g, 8.4mmol) was dissolved in aqueous NaOH (IN, 10 niL) and water added (70 mL). The mixture was heated to 37-380C and neutralized with HCl (IN) to pΗ 7.3-7.8. L-Aminoacylase (0.5 g) was added to the mixture and allowed to stir for 2 days, maintaining 37-380C and pΗ 7.3-7.8. The mixture was heated to 60 °C for another hour, concentrated to remove part of water, cooled and filtered. The filtrate was adjusted to pΗ 5.89 and filtered again. The filtrate was adjusted to pΗ 2.0 and extracted with EtOAc. The combined organic layer was dried over Na2SO4, filtered, concentrated and the residue was purified with chromatography (petroleum ether /EtOAc 1 : IEtOAc) to give R- 2-acetylamino-3-(6-chloro-5-fluoro-1H-mdol-3-yl)-propπonic acid as a light yellow solid (1.2 g, 48% yield). Step 3: R-2-acetylamino-3-(6-chloro-5-fluoro-1H-indol-3-yl)-proprionic acid (1.2 g, 4.0 mmol) was dissolved in HCl (6N, 10 mL) and the mixture heated to reflux for 4 hours, and then concentrated to dryness. Toluene (50 mL) was added to the residue and concentrated to dryness to remove water and HCl. The residue was dried under vacuum and then dissolved in MeOH (20 mL). To the solution was added dropwise SOCl2 (0.5 mL, 6.8 mmol) at 0 °C, and the mixture was stirred overnight. After removal of solvent, the residue was dissolved in THF/water (40/10 mL) and NaHCO3 (1.0 g, 11.9 mmol) was added portionwise. Upon basifϊcation, BoC2O (1.2 g, 5.5 mmol) added at 0 °C and allowed to stir at room temperature. After TLC indicated the reaction was finished, EtOAc was added and separated and the aqueous layer was extracted with EtOAc. The combined organic layers were washed with water and brine, dried with Na2SO4, filtered, concentrated and the residue was purified with chromatography (petroleum ether /EtOAc: 5/1) to give R-2-tert-butoxycarbonylamino-3-(6-chloro-5-fluoro-l/-/-indol-3-yl)-proprionic acid methyl ester 460 g, 31% yield for 3 steps).
Step 4: To a solution of R-2-tert-butoxycarbonylamino-3-(6-chloro-5-fluoro-l//-indol-3-yl)- proprionic acid methyl ester (460mg, 1.2mmol) in dry ether (20 mL) was added portionwise LiAlH4 (92 mg, 2.4 mmol) at 0 °C. The mixture was heated to reflux for 2 hours. After TLC indicated the reaction was finished, the mixture was cooled and carefully quenched with Na2SO4. The mixture was filtered and the filtrate was washed with saturated aqueous NH4Cl and water, dried with Na2SO4, filtered, concentrated to give a crude product (400 mg), which was used without further purification.
Step 5: To a solution of the crude product (400 mg, 1.2mmol) and Et3N (0.3 mL, 2.2 mmol) in CH2Cl2 (5 mL) was added MsCl (160 mg, 1.4 mmol) dropwise at 0 °C. The mixture was stirred for 2 hours at room temperature. After TLC indicated the reaction was completed, the mixture was washed with water and brine, dried with Na2SO4, filtered, concentrated and the residue was purified with chromatography (petroleum ether/EtOAc 5:1) to give methansulfonic acid (R)-2- ?ert-butoxycarbonylamino-3-(6-chloro-5-fluoro-1H-indol-3-yl)-propyl ester as a light yellow solid (300 mg, 57% yield, 2 steps)
Step 6: To a solution of mesylate (300 mg, 0.7mmol) in dry ether (20 mL) was added portionwise LiAlH4 (55 mg, 1.4 mmol) at 0 °C. The mixture was stirred at room temperature overnight. After TLC indicated the reaction was finished, the mixture was cooled and carefully quenched with Na2SO4. The mixture was filtered and the filtrate was washed with saturated aqueous NH4Cl and water, dried with Na2SO4, filtered, concentrated and the residue was purified with chromatography (petroleum ether/EtOAc 10: 1) to give [(5)-2-(6-chloro-5-fluoro-1H-indol-3-yl)- 1 -methyl-ethyl] -carbamic acid tert-butyl ester as a light yellow solid (200 mg, 87% yield).
Step 7: A solution of [(S)-2-(6-chloro-5-fluoro-1H-indol-3-yl)-l-methyl-ethyl]-carbamic acid tert-butyl ester (200 mg, 0.6 mmol) in HCl/MeOH (10 mL) was stirred at room temperature. After TLC indicated the reaction was finished, the mixture was concentrated to remove the solvent. To the residue was added EtOAc (5OmL), and the mixture was neutralized with saturated NaHCO3 to pH 8~9, and then extracted with EtOAc. The combined organic phases were dried with Na2SO4, filtered, concentrated to give a crude (S)-2-(6-chloro-5-fluoro-1H-indol-3-yl)-l- methyl-ethylamine which was used without further purification.
Step 8: To a solution of (5)-2-(6-chloro-5-fluoro-1H-indol-3-yl)-l-methyl-ethylamine (120 mg, 0.5 mmol) in EtOH (1OmL) was added 5-chloroisatin (90 mg, 0.5 mmol) and p-TsOΗ (8 mg, 0.04 mmol). The mixture was heated in a sealed tube at 1100C for 16 hours. After TLC indicated the reaction was finished, the mixture was cooled and concentrated. The residue was dissolved in EtOAc (2OmL) and washed with NaOH (IN) and brine, dried with Na2SO4, filtered, concentrated and the residue was purified with chromatography (petroleum ether/EtOAc 5:1) to give 36 (150mg, 64% yield over two steps).
Example 48 (15,3R)-5'-Chloro-3-methyl-2,3,4,9-tetrahydrospiro[β-carboline-l,3'-indol]-2'(l'JH)-one
(35)
35
Compound 35 may be prepared according to Scheme F using the same or analogous synthetic techniques and/or substituting with alternative reagents.
(lS^RVS'-Chloro-S-methyl-l^^^-tetrahydrospirotβ-carboline-l.S'-indoll-l^l'ZO-one: 1H NMR (300 MHz, DMSO-^6): δ 10.45 (s, IH), 10.42 (s, IH), 7.43 (d, J= 7.5 Hz, IH), 7.31 (dd, J = 2.1, 8.4 Hz, IH), 7.16 (d, J = 7.2 Hz, IH), 7.05-7.02 (m, 2H), 7.00-6.96 (m, IH), 6.92 (d, J = 8.1 Hz, IH), 3.98-3.86 (m, IH), 2.78 (dd, J= 3.6, 14.9 Hz, IH), 2.41 (dd, J= 4.5, 25.5 Hz, IH), 1.18 (d, J= 6.3 Hz, 3H); MS (ESI) m/z 338.0 (M+H)+.
Chiral compounds such as 36 and 37 can be prepared according to Scheme G or H using the same or analogous synthetic techniques and/or substituting with alternative reagents. Example 49
(IR^^-S'.T-Dichloro-ό-fluoro-S-methyl-l^^^-tetrahydrospiroIβ-carboline-l^'-indol]- 2\VH)-one (36)
36
35: 1H NMR (500 MHz, DMSO-Jd) δ 10.69 (s, IH), 10.51 (s, IH), 7.43 (d, J = 10.0 Hz, IH), 7.33 (dd, J= 8.4, 2.2 Hz, IH), 7.27 (d, J= 6.5 Hz, IH), 7.05 (d, J= 2.3, IH), 6.93 (d, J= 8.5 Hz, IH), 3.91 (m, IH), 3.13 (bd, J= 6.2 Hz, IH), 2.74 (dd, J= 15.0 , 3.0 Hz, IH), 2.35 (dd, J= 15.0, 10.3, IH), 1.15 (d, J= 6.0, 3H);
MS (ESI) m/z 392.0 (M+2H)+;
[α]25 D = + 255.4°
Example 50
(lS,3R)-5',7-Dichloro-6-fluoro-3-methyI-2,3,4,9-tetrahydrospiro[β-carboline-l,3'-indol]- 2'(l'H)-one (37)
37
(lS^^-S'^-Dichloro-o-fluoro-S-methyl^jS^^-tetrahydrospirojP-carboline-l-S'-indol]- 2'(l'H)-one: 1H NMR (500 MHz, CDCl3) δ 8.49 (s, IH), 7.54 (s, IH), 7.24 (d, J= 9.7 Hz, IH), 7.21 (dd, J = 8.6, 2.0 Hz, IH), 7.14 (d, J= 6.0 Hz, IH), 7.11 (d, J= 1.8, IH), 6.77 (d, J= 8.3 Hz, IH), 4.14 (m, IH), 2.89 (dd, J = 15.4, 3.7 Hz, IH), 2.49 (dd, J = 15.3, 10.5, IH), 1.68 (bs, IH), 1.29 (d, J= 6.4 Hz, 3H); MS (ESI) m/z 392.0 (M+2H)+; [α]25D -223.3°
PATENT
US 2011275613
Prior art:
(1 'R, 3'S)-5, 7'-dichloro-6'-fIuoro-3'-methyl-2', 3',4', 9'-tetrahydrospiro[indoline-3, 1 - pyrido[3,4-b]indol]-2-one (eg. a compound of formula (IV), which comprises a spiroindolone moiety) and a 6-steps synthetic method for preparing, including known chiral amine intermediate compound (MA) are known (WO 2009/132921 ):
he
present invention relates to processes for the preparation of
spiroindolone compounds, such as (1'R,3'S)-5,
7'-dichloro-6'-fIuoro-3'-methyl-2',3',4',9'- tetrahydrospiro[indoline-3,
1 '-pyhdo[3.4-b]indol]-2-one.
(1 'R, 3'S)-5, 7'-dichloro-6'-fluoro-3'-methyl-2', 3',4 9'-tetrahydrospiro[indoline-3, 1 '- pyrido[3, 4-b]indol]-2-one is useful in the treatment and/or prevention of infections such as those caused by Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, Trypanosoma cruzi and parasites of the Leishmania genus such as, for example, Leishmania donovani., and it has the following structure:
(IVA)
(1 'R, 3'S)-5, 7'-dichloro-6'-fluoro-3'-methyl-2 3', 4', 9'-tetrahydrospiro[indoline-3, 1 - pyhdo[3, 4-b]indol]-2-one and a synthesis thereof are described in WO 2009/132921 Al in particular in Example 49 therein.
(1 'R, 3'S)-5, 7'-dichloro-6'-fluoro-3'-methyl-2', 3',4 9'-tetrahydrospiro[indoline-3, 1 '- pyrido[3, 4-b]indol]-2-one is useful in the treatment and/or prevention of infections such as those caused by Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, Trypanosoma cruzi and parasites of the Leishmania genus such as, for example, Leishmania donovani., and it has the following structure:
(IVA)
(1 'R, 3'S)-5, 7'-dichloro-6'-fluoro-3'-methyl-2 3', 4', 9'-tetrahydrospiro[indoline-3, 1 - pyhdo[3, 4-b]indol]-2-one and a synthesis thereof are described in WO 2009/132921 Al in particular in Example 49 therein.
Example 10: Process for Conversion of Compound (IA) to Compound (IIA) in 30g Scale
458.97
152.48g /so-propylamine hydrochloride and 0.204g pyridoxalphosphate monohydrate were dissolved in 495ml water while stirring. To this yellow clear solution a solution of 30. Og ketone in 85ml poly ethylene glycol (average mol weight 200) within 15 minutes. Upon addition the ketone precipitates as fine particles which are evenly distributed in the reaction media. To the suspension 180ml triethanolamine buffer (0.1 mol/l, pH 7) were added and the pH was adjusted to 7 by additon of aqueous sodium hydroxide solution (1 mol/l). The reaction mixture is heated to 50°C and a solution of 1.62g transaminase SEQ ID NO: 134 dissolved in 162ml triethanolamine buffer (0 1 mol/l, pH 7) is added. The reaction mixture is continiously kept at pH 7 by addition of 1 mol/l aqueous sodium hydroxide solution. The reaction mixture is stirred 24h at 50°C and a stream of Nitrogen is blown over the surface of the reaction mixture to strip off formed acetone. The reaction mixture is then cooled to 25°C and filtered over a bed of cellulose flock. The pH of the filtrate is adjusted to «1 by addition of concentrated sulfuric acid. The acidified filtrated is extracted with 250 ml /so-Propyl acetate. The layers are separated and the pH of the aqueous phase is adjusted to ¾10 by additon of concentrated aqueous sodium hydroxide solution. The basified aqueous phase is extracted with /'so-propyl acetate. The layers are seperated and the organic phase is washed with 100 ml water. The organic phase is concentrated by distillation to 2/3 of its origin volume. In a second reactor 33.98g (+)- camphor sulfonic acid is dissolved in 225 ml /'so-propyl acetate upon refluxing and the concentrated organic phase is added within 10 minutes. After complete addition the formed thin suspension is cooled to 0°C within 2 hours and kept at 0°C for 15 hours. The precipitated amine-(+)-camphor sulfonate salt is filtered, washed with 70 ml /so-propyl acetate and dried at 40°C in vaccuum yielding 51.57g of colourless crystals (84.5% yield t.q.)
Analytical Data
IR:
v (crn 1)=3296, 3061 , 2962, 2635, 2531 , 2078, 1741 , 1625, 1577, 1518, 1461 , 1415, 1392, 1375, 1324, 1302, 1280, 1256, 1226, 1 170, 1 126, 1096, 1041 , 988, 966, 937, 868, 834, 814, 790, 766, 746, 719, 669, 615.
LC-MS (ESI +):
Ammonium ion: m/z =227 ([M+H]), 268 ([M+H+CH3CN]), 453 ([2M+H]).
Camphorsulfonate ion: m/z =250 ([M+NH4]), 482 ([2M+NH4]).
LC-MS (ESI -):
Camphorsulfonate ion: m/z=231 ([M-H]), 463 ([2M-H]).
1H-NMR (DMSO-d6, 400 MHz):
1 1.22 (br. s., 1 H), 7.75 (br. s., 3H), 7.59 (d, J = 10.3 Hz, 1 H), 7.54 (d, J = 6.5 Hz, 1 H), 7.36 (d, J = 2.3 Hz, 1 H), 3.37 - 3.50 (m, 1 H), 2.98 (dd, J = 14.3, 5.8 Hz, 1 H), 2.91 (d, J = 14.8 Hz, 1 H), 2,81 (dd, J = 14.3, 8.0 Hz, 1 H), 2.63 - 2.74 (m, 1 H), 2.41 (d, J = 14.6 Hz, 1 H), 2.24 (dt, J = 18.3, 3.8 Hz, 1 H), 1 .94 (t, J = 4.4 Hz, 1 H), 1.86 (dt, J = 7.4, 3 6 Hz, 1 H), 1.80 (d, J = 18.1 Hz, H), 1.23 - 1 .35 (m, 2H), 1.15 (d, J = 6.3 Hz, 3H), 1.05 (s, 3H), 0.74 (s, 3H)
Free Amine (obtained by evaporatig the iso-Propylacetate layer after extraction of the basified aqueous layer):
1H NMR (400MHz, DMSO-d6): 11 .04 (br. s., 1 H), 7.50 (d, J = 10.5 Hz, 1 H), 7.48 (d, J = 6.5 Hz, 1 H), 7.25 (s, 1 H), 3.03 (sxt, J = 6.3 Hz, 1 H), 2.61 (dd, J - 14.3, 6.5 Hz, 1 H), 2.57 (dd, J = 14.1 , 6.5 Hz, 1 H), 1.36 (br. s., 2H), 0.96 (d, J = 6.3 Hz, 3H)
Example 11: Process for Conversion of Compound (HA) to Compound (IVB)
3. solvent exchange to TP
13.62 g 5-chloroisatin is suspended in 35 ml /so-propanol and 2.3 g triethyl amine is added. The suspension is heated to reflux and a solution of 34.42g amine-(+)-camphor sulfonate salt dissolved in 300 ml /'so-propanol is added within 50 minutes. The reaction mixture is stirred at reflux for 17 hours. The reaction mixture is cooled to 75°C and 17.4g (+)-camphorsulfonic acid are added to the reaction mixture. Approximately 300 ml /so- propanol are removed by vacuum distillation. Distilled off /so-propanol is replaced by iso- propyl acetate and vacuum distillation is continued. This is distillation is repeated a second time. To the distillation residue 19 ml ethanol and 265 ml ethyl acetate is added and the mixture is heated to reflux. The mixture is cooled in ramps to 0°C and kept at 0°C for 24 hours. The beige to off white crystals are filtered off, washed with 3 portions (each 25 ml) precooled (0°C) ethylacetate and dried in vacuum yielding 40.3 g beige to off white crystals. (86.3% yield t.q.)
IR:
v (crrr)= 3229, 3115, 3078, 3052, 2971 , 2890, 2841. 2772. 2722, 2675, 2605, 2434. 1741 , 1718, 1621 , 1606, 1483, 1460, 1408, 1391 , 1372, 1336, 1307, 1277, 1267, 1238, 1202, 1 184, 1 162, 1 149, 1 128, 1067, 1036, 987, 973, 939, 919, 896, 871 , 857, 843, 785, 771 , 756, 717, 690, 678, 613.
LC-MS (ESI +):
Ammonium ion: m/z =390 ([M+H]), 431 ([M+H+CH3CN]) Camphorsulfonate ion: m/z =250 ([M+NH4]), 482 ([2M+NH4])
LC-MS (ESI -):
Camphorsulfonate ion: m/z=231 ([M-H]), 463 ([2M-H])
1H NMR (DMSO-d6, 600 MHz):
11.49 (s, 1 H), 1 1.23 (s, 1 H), 10.29 - 10.83 (m, 1 H), 9.78 - 10.31 (m, 1 H), 7.55 - 7.60 (m, 2H), 7.52 (s, 1 H), 7.40 (d, J = 6.2 Hz, H), 7.16 (d, J = 8.8 Hz, 1 H), 4.52 - 4.63 (m, 1 H). 3.20 (dd, J = 16.3, 4.2 Hz, 1 H), 2.96 (dd, J = 16.1 , 11.3 Hz, 1 H), 2.90 (d, J = 15.0 Hz, 1 H), 2.56 - 2.63 (m, 1 H), 2.39 (d, J = 14.6 Hz, 1 H), 2.21 (dt, J = 18.0, 3.8 Hz, 1 H), 1.89 - 1.93 (m, 1 H), 1.81 (ddd, J = 15.3, 7.8, 3.7 Hz, 1 H), 1.76 (d, J = 18.3 Hz, 1 H), 1 .53 (d, J = 6.6 Hz, 3H), 1.20 - 1.33 (m, 2H), 0.98 (s, 3H), 0.70 (s, 3H)
Example 12: Process for Preparing a Compound of formula (IVA) 1/z Hydrate
mw622.54 .............................................................................mw399.25
In a 750ml reactor with impeller stirrer 50g of compound (IVB) salt were dissolved in 300ml Ethanol (ALABD) and 100 ml deionised Water (WEM). The clear, yellowish sollution was heated to 58°C internal temperature. To the solution 85 g of a 10% aqueous sodium carbonate solution was added within 10 minutes. The clear solution was particle filtered into a second reaction vessel. Vessel and particle filter were each rinsed with 25 ml of a mixture of ethanohwater (3:1 v/v) in the second reaction vessel. The combined particle filtered solution is heated to 58°C internal temperature and 200ml water (WEM) were added dropwise within 15 minutes. Towards the end of the addition the solution gets turbid.
The mixture is stirred for 10 minutes at 58°C internal temperature and is then cooled slowely to room temperature within 4hours 30 minutes forming a thick, well stirable white suspension. To the suspension 200 ml water are added and the mixture is stirred for additional 15hours 20 minutes at room temperature. The suspension is filtered and the filter cake is washed twice with 25 ml portions of a mixture of ethanohwater 9: 1 (v/v). The colourless crystals are dried at 60°C in vacuum yielding 26.23g (=91.2% yield). H NMR (400 MHz, DMSO-d6)
0.70 (s, 1H), 10.52 (s, 1H), 7.44 (d, J = 10.0 Hz, 1H), 7.33 (dd, J = 8.4, 2.1 Hz, 1H),.26 (d, J = 6.5 Hz, 1H), 7.05 (d, J = 2.3 Hz, 1H), 6.93 (d, J = 8.3 Hz, 1H), 3.83 - 4.00 (m,H), 3.13 (d, J = 6.0 Hz, 1H), 2.77 (dd, J = 15.1, 3.8 Hz, 1H), 2.38 (dd, J = 15.1, 10.5 Hz,H), 1.17 (d, J = 6.3 Hz, 3H).
458.97
152.48g /so-propylamine hydrochloride and 0.204g pyridoxalphosphate monohydrate were dissolved in 495ml water while stirring. To this yellow clear solution a solution of 30. Og ketone in 85ml poly ethylene glycol (average mol weight 200) within 15 minutes. Upon addition the ketone precipitates as fine particles which are evenly distributed in the reaction media. To the suspension 180ml triethanolamine buffer (0.1 mol/l, pH 7) were added and the pH was adjusted to 7 by additon of aqueous sodium hydroxide solution (1 mol/l). The reaction mixture is heated to 50°C and a solution of 1.62g transaminase SEQ ID NO: 134 dissolved in 162ml triethanolamine buffer (0 1 mol/l, pH 7) is added. The reaction mixture is continiously kept at pH 7 by addition of 1 mol/l aqueous sodium hydroxide solution. The reaction mixture is stirred 24h at 50°C and a stream of Nitrogen is blown over the surface of the reaction mixture to strip off formed acetone. The reaction mixture is then cooled to 25°C and filtered over a bed of cellulose flock. The pH of the filtrate is adjusted to «1 by addition of concentrated sulfuric acid. The acidified filtrated is extracted with 250 ml /so-Propyl acetate. The layers are separated and the pH of the aqueous phase is adjusted to ¾10 by additon of concentrated aqueous sodium hydroxide solution. The basified aqueous phase is extracted with /'so-propyl acetate. The layers are seperated and the organic phase is washed with 100 ml water. The organic phase is concentrated by distillation to 2/3 of its origin volume. In a second reactor 33.98g (+)- camphor sulfonic acid is dissolved in 225 ml /'so-propyl acetate upon refluxing and the concentrated organic phase is added within 10 minutes. After complete addition the formed thin suspension is cooled to 0°C within 2 hours and kept at 0°C for 15 hours. The precipitated amine-(+)-camphor sulfonate salt is filtered, washed with 70 ml /so-propyl acetate and dried at 40°C in vaccuum yielding 51.57g of colourless crystals (84.5% yield t.q.)
Analytical Data
IR:
v (crn 1)=3296, 3061 , 2962, 2635, 2531 , 2078, 1741 , 1625, 1577, 1518, 1461 , 1415, 1392, 1375, 1324, 1302, 1280, 1256, 1226, 1 170, 1 126, 1096, 1041 , 988, 966, 937, 868, 834, 814, 790, 766, 746, 719, 669, 615.
LC-MS (ESI +):
Ammonium ion: m/z =227 ([M+H]), 268 ([M+H+CH3CN]), 453 ([2M+H]).
Camphorsulfonate ion: m/z =250 ([M+NH4]), 482 ([2M+NH4]).
LC-MS (ESI -):
Camphorsulfonate ion: m/z=231 ([M-H]), 463 ([2M-H]).
1H-NMR (DMSO-d6, 400 MHz):
1 1.22 (br. s., 1 H), 7.75 (br. s., 3H), 7.59 (d, J = 10.3 Hz, 1 H), 7.54 (d, J = 6.5 Hz, 1 H), 7.36 (d, J = 2.3 Hz, 1 H), 3.37 - 3.50 (m, 1 H), 2.98 (dd, J = 14.3, 5.8 Hz, 1 H), 2.91 (d, J = 14.8 Hz, 1 H), 2,81 (dd, J = 14.3, 8.0 Hz, 1 H), 2.63 - 2.74 (m, 1 H), 2.41 (d, J = 14.6 Hz, 1 H), 2.24 (dt, J = 18.3, 3.8 Hz, 1 H), 1 .94 (t, J = 4.4 Hz, 1 H), 1.86 (dt, J = 7.4, 3 6 Hz, 1 H), 1.80 (d, J = 18.1 Hz, H), 1.23 - 1 .35 (m, 2H), 1.15 (d, J = 6.3 Hz, 3H), 1.05 (s, 3H), 0.74 (s, 3H)
Free Amine (obtained by evaporatig the iso-Propylacetate layer after extraction of the basified aqueous layer):
1H NMR (400MHz, DMSO-d6): 11 .04 (br. s., 1 H), 7.50 (d, J = 10.5 Hz, 1 H), 7.48 (d, J = 6.5 Hz, 1 H), 7.25 (s, 1 H), 3.03 (sxt, J = 6.3 Hz, 1 H), 2.61 (dd, J - 14.3, 6.5 Hz, 1 H), 2.57 (dd, J = 14.1 , 6.5 Hz, 1 H), 1.36 (br. s., 2H), 0.96 (d, J = 6.3 Hz, 3H)
Example 11: Process for Conversion of Compound (HA) to Compound (IVB)
3. solvent exchange to TP
13.62 g 5-chloroisatin is suspended in 35 ml /so-propanol and 2.3 g triethyl amine is added. The suspension is heated to reflux and a solution of 34.42g amine-(+)-camphor sulfonate salt dissolved in 300 ml /'so-propanol is added within 50 minutes. The reaction mixture is stirred at reflux for 17 hours. The reaction mixture is cooled to 75°C and 17.4g (+)-camphorsulfonic acid are added to the reaction mixture. Approximately 300 ml /so- propanol are removed by vacuum distillation. Distilled off /so-propanol is replaced by iso- propyl acetate and vacuum distillation is continued. This is distillation is repeated a second time. To the distillation residue 19 ml ethanol and 265 ml ethyl acetate is added and the mixture is heated to reflux. The mixture is cooled in ramps to 0°C and kept at 0°C for 24 hours. The beige to off white crystals are filtered off, washed with 3 portions (each 25 ml) precooled (0°C) ethylacetate and dried in vacuum yielding 40.3 g beige to off white crystals. (86.3% yield t.q.)
IR:
v (crrr)= 3229, 3115, 3078, 3052, 2971 , 2890, 2841. 2772. 2722, 2675, 2605, 2434. 1741 , 1718, 1621 , 1606, 1483, 1460, 1408, 1391 , 1372, 1336, 1307, 1277, 1267, 1238, 1202, 1 184, 1 162, 1 149, 1 128, 1067, 1036, 987, 973, 939, 919, 896, 871 , 857, 843, 785, 771 , 756, 717, 690, 678, 613.
LC-MS (ESI +):
Ammonium ion: m/z =390 ([M+H]), 431 ([M+H+CH3CN]) Camphorsulfonate ion: m/z =250 ([M+NH4]), 482 ([2M+NH4])
LC-MS (ESI -):
Camphorsulfonate ion: m/z=231 ([M-H]), 463 ([2M-H])
1H NMR (DMSO-d6, 600 MHz):
11.49 (s, 1 H), 1 1.23 (s, 1 H), 10.29 - 10.83 (m, 1 H), 9.78 - 10.31 (m, 1 H), 7.55 - 7.60 (m, 2H), 7.52 (s, 1 H), 7.40 (d, J = 6.2 Hz, H), 7.16 (d, J = 8.8 Hz, 1 H), 4.52 - 4.63 (m, 1 H). 3.20 (dd, J = 16.3, 4.2 Hz, 1 H), 2.96 (dd, J = 16.1 , 11.3 Hz, 1 H), 2.90 (d, J = 15.0 Hz, 1 H), 2.56 - 2.63 (m, 1 H), 2.39 (d, J = 14.6 Hz, 1 H), 2.21 (dt, J = 18.0, 3.8 Hz, 1 H), 1.89 - 1.93 (m, 1 H), 1.81 (ddd, J = 15.3, 7.8, 3.7 Hz, 1 H), 1.76 (d, J = 18.3 Hz, 1 H), 1 .53 (d, J = 6.6 Hz, 3H), 1.20 - 1.33 (m, 2H), 0.98 (s, 3H), 0.70 (s, 3H)
Example 12: Process for Preparing a Compound of formula (IVA) 1/z Hydrate
mw622.54 .............................................................................mw399.25
In a 750ml reactor with impeller stirrer 50g of compound (IVB) salt were dissolved in 300ml Ethanol (ALABD) and 100 ml deionised Water (WEM). The clear, yellowish sollution was heated to 58°C internal temperature. To the solution 85 g of a 10% aqueous sodium carbonate solution was added within 10 minutes. The clear solution was particle filtered into a second reaction vessel. Vessel and particle filter were each rinsed with 25 ml of a mixture of ethanohwater (3:1 v/v) in the second reaction vessel. The combined particle filtered solution is heated to 58°C internal temperature and 200ml water (WEM) were added dropwise within 15 minutes. Towards the end of the addition the solution gets turbid.
The mixture is stirred for 10 minutes at 58°C internal temperature and is then cooled slowely to room temperature within 4hours 30 minutes forming a thick, well stirable white suspension. To the suspension 200 ml water are added and the mixture is stirred for additional 15hours 20 minutes at room temperature. The suspension is filtered and the filter cake is washed twice with 25 ml portions of a mixture of ethanohwater 9: 1 (v/v). The colourless crystals are dried at 60°C in vacuum yielding 26.23g (=91.2% yield). H NMR (400 MHz, DMSO-d6)
0.70 (s, 1H), 10.52 (s, 1H), 7.44 (d, J = 10.0 Hz, 1H), 7.33 (dd, J = 8.4, 2.1 Hz, 1H),.26 (d, J = 6.5 Hz, 1H), 7.05 (d, J = 2.3 Hz, 1H), 6.93 (d, J = 8.3 Hz, 1H), 3.83 - 4.00 (m,H), 3.13 (d, J = 6.0 Hz, 1H), 2.77 (dd, J = 15.1, 3.8 Hz, 1H), 2.38 (dd, J = 15.1, 10.5 Hz,H), 1.17 (d, J = 6.3 Hz, 3H).
PAPER
Journal of Medicinal Chemistry, 2010 , vol. 53, 14 p. 5155 - 5164
(1R,3S)-5′,7-Dichloro-6-fluoro-3-methyl-2,3,4,9-tetrahydrospiro[β-carboline-1,3′-indol]-2′(1′H)-one (19a)
1H NMR (500 MHz, DMSO-d6): δ 10.69 (s, 1H), 10.51 (s, 1H), 7.43 (d, J = 10.0 Hz, 1H), 7.33 (dd, J = 8.0, 2.2 Hz, 1H), 7.27 (d, J = 6.5 Hz, 1H), 7.05 (d, J = 2.3 Hz, 1H), 6.93 (d, J = 8.5 Hz, 1H), 3.91 (m, 1H), 3.13 (bd, J = 6.2 Hz, 1H), 2.74 (dd, J = 15.0, 3.0 Hz, 1H), 2.35 (dd, J = 15.0, 10.3 Hz, 1H), 1.15 (d, J = 6.0 Hz, 3H). MS (ESI) m/z 392.0 (M + 2H)+; [α]D25 = +255.4° (c = 0.102 g/L, methanol).
CLIPS
Z.Zhang, WO 2007 / 104714,2007).
[0008] (2) year 2008 Roche pharmaceutical company disclosed a spiro [oxindole - cyclohexenone] skeleton biomedicine, PCT International Application No. W02008 / 055812. It also announced the preparation of anti-cancer agents and antagonists of the application of the compound is used as the interaction with MDM2 (reference:. Liu, J.-J; Zhang, Z; (Hoffmann-LaRoche AG), PCT Int App 1. . W02008 / 055812, 2008), its structural formula is as follows:
[0009]
(3) Melchiorre research group abroad chiral amines and o-fluoro-3-benzyl benzoate as catalyst methylene-indole-2-one (3-benzylideneindolin-2-one, CAS Number: 3359-49- 7) with α, β - unsaturated ketone synthesis of chiral spiro [cyclohexane _1,3'- indole] _2,4 '- dione [s pir0 [cycl0hexane-l, 3' -indoline] - 2 ', 4-diones] compounds (see:.. Bencivenni, G; ffu, LY; Mazzanti, A .; Giannichi, B.; Pesciaioli, F; Song, Μ P.; Bartoli, G.; Melchiorre, P .... .Angew Chem Int Ed 2009,48,7200), the structure of the total formula is as follows:
(4) Gong Flow column team found to cyclohexanediamine derived Bronsted acid - a bifunctional catalyst Lewis base catalysis of 3-benzyl-methylene-indole-2-one and α, β- unsaturated 1,3 tandem reaction dicarbonyl compound (Nazarov reagent) can be obtained with high stereoselectivity chiral spiro [cyclohexane _1,3'- indol] -2 ', 4-dione [spiro [cyclohexane-l, 3 '-indoline] -2', 4-diones] compounds; and by this method successfully synthesized 7 Roche pharmaceutical companies to develop chiral anti-tumor agents (see: Q Wei, L -Z Gong, Org Lett 2010..... , 12, 1008.).
(5) Wang Lixin research group recently reported that primary amines derived from cinchona alkaloids and Bronsted acid as catalyst N- protected indolone compounds and double Michael addition reaction of diketene generate hand spiro [cyclohexane-1, 3'-indol] -2 ', 4-dione [spiro [cyclohexane-l, 3' -indoline] -2 ', 4-diones] type of tx ^ (: L. -L. Wang, L. Peng, J. -F. Bai, L. -N. Jia, X. -Y. Luo, QC Huang, L. -X. Wang, Chem. Commum. 2011,47, 5593.).
[0008] (2) year 2008 Roche pharmaceutical company disclosed a spiro [oxindole - cyclohexenone] skeleton biomedicine, PCT International Application No. W02008 / 055812. It also announced the preparation of anti-cancer agents and antagonists of the application of the compound is used as the interaction with MDM2 (reference:. Liu, J.-J; Zhang, Z; (Hoffmann-LaRoche AG), PCT Int App 1. . W02008 / 055812, 2008), its structural formula is as follows:
[0009]
(3) Melchiorre research group abroad chiral amines and o-fluoro-3-benzyl benzoate as catalyst methylene-indole-2-one (3-benzylideneindolin-2-one, CAS Number: 3359-49- 7) with α, β - unsaturated ketone synthesis of chiral spiro [cyclohexane _1,3'- indole] _2,4 '- dione [s pir0 [cycl0hexane-l, 3' -indoline] - 2 ', 4-diones] compounds (see:.. Bencivenni, G; ffu, LY; Mazzanti, A .; Giannichi, B.; Pesciaioli, F; Song, Μ P.; Bartoli, G.; Melchiorre, P .... .Angew Chem Int Ed 2009,48,7200), the structure of the total formula is as follows:
(4) Gong Flow column team found to cyclohexanediamine derived Bronsted acid - a bifunctional catalyst Lewis base catalysis of 3-benzyl-methylene-indole-2-one and α, β- unsaturated 1,3 tandem reaction dicarbonyl compound (Nazarov reagent) can be obtained with high stereoselectivity chiral spiro [cyclohexane _1,3'- indol] -2 ', 4-dione [spiro [cyclohexane-l, 3 '-indoline] -2', 4-diones] compounds; and by this method successfully synthesized 7 Roche pharmaceutical companies to develop chiral anti-tumor agents (see: Q Wei, L -Z Gong, Org Lett 2010..... , 12, 1008.).
(5) Wang Lixin research group recently reported that primary amines derived from cinchona alkaloids and Bronsted acid as catalyst N- protected indolone compounds and double Michael addition reaction of diketene generate hand spiro [cyclohexane-1, 3'-indol] -2 ', 4-dione [spiro [cyclohexane-l, 3' -indoline] -2 ', 4-diones] type of tx ^ (: L. -L. Wang, L. Peng, J. -F. Bai, L. -N. Jia, X. -Y. Luo, QC Huang, L. -X. Wang, Chem. Commum. 2011,47, 5593.).
| WO2009132921A1 * | Apr 1, 2009 | Nov 5, 2009 | Novartis Ag | Spiro-indole derivatives for the treatment of parasitic diseases |
| WO2010081053A2 * | Jan 8, 2010 | Jul 15, 2010 | Codexis, Inc. | Transaminase polypeptides |
| WO2012007548A1 * | Jul 14, 2011 | Jan 19, 2012 | Dsm Ip Assets B.V. | (r)-selective amination |
| AT507050A1 * | Title not available | |||
| EP0036741A2 * | Mar 17, 1981 | Sep 30, 1981 | THE PROCTER & GAMBLE COMPANY | Phosphine compounds, transition metal complexes thereof and use thereof as chiral hydrogenation catalysts |
| EP0120208A2 * | Jan 24, 1984 | Oct 3, 1984 | Degussa Aktiengesellschaft | Microbiologically produced L-phenylalanin-dehydrogenase, process for obtaining it and its use |
| EP0135846A2 * | Aug 31, 1984 | Apr 3, 1985 | Genetics Institute, Inc. | Production of L-amino acids by transamination |
| GB974895A * | Title not available | |||
| US3282959 * | Mar 21, 1962 | Nov 1, 1966 | Parke Davis & Co | 7-chloro-alpha-methyltryptamine derivatives |
| US4073795 * | Jun 22, 1976 | Feb 14, 1978 | Hoffmann-La Roche Inc. | Synthesis of tryptophans |
| WO2005009370A2 * | Jul 22, 2004 | Feb 3, 2005 | Pharmacia Corp | Beta-carboline compounds and analogues thereof and their use as mitogen-activated protein kinase-activated protein kinase-2 inhibitors |
| EP0466548A1 * | Jun 27, 1991 | Jan 15, 1992 | Adir Et Compagnie | 1,2,3,4,5,6-Hexahydroazepino[4,5-b]indole and 1,2,3,4-tetrahydro-beta-carbolines, processes for their preparation, and pharmaceutical compositions containing them |

Исследовательская группа Элизабет Винцелер (Elizabeth A. Winzeler) разработала новый препарат, первоначально проведя скрининг библиотеки, состоящей из 12000 соединений, а затем получив производные наиболее перспективных кандидатов. В результате долгой работы исследователи отобрали единственное соединение спироиндолоновой структуры, получившее регистрационный номер NITD609. В случае успешного прохождения экспертизы фармакологических и токсикологических свойств нового соединения исследователи надеются приступить к первой фазе его клинических испытаний уже в конце этого года.
Было обнаружено, что NITD609 быстро останавливает белковый синтез в организме возбудителя малярии, ингибируя ген аденозинтрифосфатазы, ответственной за транспорт катионов через мембрану клетки возбудителя. То, что механизм действия нового соединения отличается от механизма, характерного для других средств лечения малярии, объясняет причины успешного действия нового препарата в том числе и против штаммов малярии, выработавших резистентность.
HPLC
Analyte quantization was performed byLC/MS/MS. Liquid chromatography was performed using an Agilent
1100 HPLC system(Santa Clara, CA), with the Agilent Zorbax XDB Phenyl (3.5μ, 4.6 x75 mm) column at
an oven temperature of 35 °C, coupled with a QTRAP4000 triple quadruple mass
spectrometer
(Applied Biosystems, Foster City, CA). Instrumentcontrol and
dataacquisition were performed using Applied Biosystems software Analyst
1.4.2. Themobile phases used were A: water:acetic acid (99.8:0.2, v/v)
and B: acetonitrile:aceticacid (99.8:0.2, v/v), using a gradient, with
flow rate of 1.0 mL/min, and run time of 5minutes. Under these
conditions the retention time of9a
was 3.2 minutes.
Compounddetection on the mass spectrometer was performed in
electrospraypositive ionizationmode and utilized multiple reaction
monitoring (MRM) for specificity (9atransitions338.3/295.1, 338.3/259.2)
together with their optimized MS parameters. The lower limitof
quantification for9awas 70 ng/mL.
Extraction and LCMS analysis
of 20a.Plasma samples were extracted withacetonitrile:methanol-acetic
acid (90:9.8:0.2 v/v) for the analyte and internal standard(17a) using a
3.6 to 1 extractant to plasma ratio. Analyte quantitation was performed
by
LC/MS/MS. Liquid chromatography was performed using an
Agilent1100 HPLC systemS7(Santa Clara, CA), with the Agilent Zorbax
XDB-Phenyl (3.5μ, 4.6x75mm) column atan oven temperature of 45 °C
coupled with a QTRAP 4000 triple quadruple massSpectrometer (Applied
Biosystems, Foster City, CA). Instrumentcontrol and dataacquisition were
performed using Applied Biosystems software Analyst 1.4.2. Themobile
phases used were A: water:acetic acid (99.8:0.2, v/v) and B:
methanol:acetic acid
(99.8:0.2, v/v), using gradient elution conditions with a flow rate of 1.0 mL/min and a runtime of 6 minutes
References
- "NITD 609". Medicines for Malaria Venture.
- Rottmann M, McNamara C, Yeung BK, Lee MC, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, González-Páez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT (2010). "Spiroindolones, a potent compound class for the treatment of malaria". Science329 (5996): 1175–80. doi:10.1126/science.1193225. PMC 3050001. PMID 20813948.
 | |
| Names | |
|---|---|
| IUPAC name
(1R,3S)-5’,7-Dichloro-6-fluoro-3-methyl-spiro[2,3,4,9-tetrahydropyrido[3,4-b]indole-1,3’-indoline]-2’-one
| |
| Identifiers | |
| 1193314-23-6 | |
| ChemSpider | 24662493 |
| Jmol interactive 3D | Image |
| PubChem | 44469321 |
| Properties | |
| C19H14Cl2FN3O | |
| Molar mass | 390.24 g·mol−1 |
C[C@H]1Cc2c3cc(c(cc3[nH]c2[C@]4(N1)c5cc(ccc5NC4=O)Cl)Cl)F
//////////
SEE.........https://newdrugapprovals.org/2014/12/27/nitd609/
++++++++++++++++