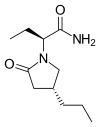
BRIVARACETAM, UCB-34714
(2S)-2-[(4R)-2-oxo-4-propylpyrrolidin-1-yl]butanamide
(2S)-2-[(4R)-2-Oxo-
1-Pyrrolidineacetam
CAS 357336-20-0
| Molecular Formula: | C11H20N2O2 |
|---|---|
| Molecular Weight: | 212.2887 g/mol |
UNII-U863JGG2IA
UCB; For the treatment of partial onset seizures related to epilepsy, Approved February 2016
Brivaracetam, the 4-n-propyl analog of levetiracetam, is a racetam derivative with anticonvulsant properties.[1][2] Brivaracetam is believed to act by binding to the ubiquitous synaptic vesicle glycoprotein 2A (SV2A).[3] Phase II clinical trials in adult patients with refractory partial seizures were promising. Positive preliminary results from stage III trials have been recorded,[4][5] along with evidence that it is around 10 times more potent[6] for the prevention of certain types of seizure in mouse models than levetiracetam, of which it is an analogue.
On 14 January 2016, the European Commission,[7] and on 18 February 2016, the USFDA[8] approved brivaracetam under the trade nameBriviact (by UCB). The launch of this anti-epileptic is scheduled for the first quarter of that year. Currently, brivaracetam is still not approved in other countries like Australia, Canada and Switzerland.
Brivaracetam was approved by European Medicine Agency (EMA) on Jan 14, 2016 and approved by the U.S. Food and Drug Administration (FDA) on Feb 18, 2016. It was developed and marketed as Briviact® by UCB in EU/US.
Brivaracetam is a selective high-affinity synaptic vesicle protein 2A ligand, as an adjunctive therapy in the treatment of partial-onset seizures with or without secondary generalization in adult and adolescent patients from 16 years of age with epilepsy.
Briviact® is available in three formulations, including film-coated tablets, oral solution and solution for injection/infusion. And it will be available as 10 mg, 25 mg, 50 mg, 75 mg and 100 mg film-coated tablets, a 10 mg/ml oral solution, and a 10 mg/ml solution for injection/infusion. The recommended starting dose is either 25 mg twice a day or 50 mg twice a day, depending on the patient’s condition. The dose can then be adjusted according to the patient’s needs up to a maximum of 100 mg twice a day. Briviact can be given by injection or by infusion (drip) into a vein if it cannot be given by mouth.
European Patent No. 0 162 036 Bl discloses compound (S)-α-ethyl-2-oxo-l- pyrrolidine acetamide, which is known under the International Non-proprietary Name of Levetiracetam.
Levetiracetam
Levetiracetam is disclosed as a protective agent for the treatment and prevention of hypoxic and ischemic type aggressions of the central nervous system in European patent EP 0 162 036 Bl. This compound is also effective in the treatment of epilepsy.
The preparation of Levetiracetam has been disclosed in European Patent No. 0 162 036 and in British Patent No. 2 225 322.
International patent application having publication number WO 01/62726 discloses 2-oxo-l -pyrrolidine derivatives and methods for their preparation. It particularly discloses compound (2S)-2-[(4R)-2-oxo-4-propyl-pyrrolidin-l-yl] butanamide known under the international non propriety name of brivaracetam.
Brivaracetam
International patent application having publication number WO 2005/121082 describes a process of preparation of 2-oxo-l -pyrrolidine derivatives and particularly discloses a process of preparation of (2S)-2-[(4S)-4-(2,2-difluorovinyl)-2-oxo-pyrrolidin-l- yl]butanamide known under the international non propriety name of seletracetam.
Seletracetam
Kenda et al., in J. Med. Chem. 2004, 47, 530-549, describe processes of preparation of 2-oxo-l -pyrrolidine derivatives and particularly discloses compound 1-((1S)-I- carbamoyl-propyl)-2-oxo-pyrrolidone-3-carboxylic acid as a synthetic intermediate.
WO2005028435
CLIPS
Find better ways to make old and new epilepsy drugs. J. Surtees and co-inventors disclose alternative processes for making active pharmaceutical ingredients (APIs) that are used to treat epilepsy and seizures. One compound that can be prepared by their processes is the established drug levetiracetam (1, Figure 1), marketed under the trade name Keppra. Because 1 is now off-patent, there is obvious interest in new drugs.
The inventors also claim that seletracetam (2) and brivaracetam (3) (Figure 2) can be prepared by their processes. These drugs are apparently much more active than 1.
All of the drugs are used as single isomers, so a stereoselective synthesis is desirable. The inventors describe two routes for preparing the molecules; the first, shown in Figure 1, is the synthesis of 1 by the reaction between pyrrolidone (4) and chiral bromo amide 5 in the presence of a base. GC analysis showed that the conversion is 40.3% and that the product contains 51% of the (S)-enantiomer and 49% of the (R)-isomer. No details of their separation are given, although the use of chiral HPLC is discussed.
The same reaction is used to prepare derivative 6 of 1. Compound 7 is prepared from the corresponding hydroxy ester and then condensed with 4 to give 6. Chiral HPLC showed that the product is a mixture of 89.3% (S)-enantiomer 6 and 10.7% of its (R)-isomer.
The inventors do not describe the detailed preparation of 2, but they report that acid 8 is prepared in 41% yield from pyrrolidone 9 and acid10 in the presence of NaH (Figure 2). Ammonolysis of 8 produces 2; no reaction details are provided.
In a reaction similar to the preparation of 8, acid 11 is prepared from 10 and pyrrolidone 12. The product is isolated in 77% yield and can be converted to 3 by ammonolysis. Again, no details are provided for this reaction.
The second route for preparing the substituted pyrrolidones does not start with simple pyrrolidones and is the subject of additional claims. The route involves a cyclization reaction, shown in Figure 3. The preparation of enantiomer 13 begins with the reaction of racemic salt 14and optically pure bromo ester 15. This step produces intermediate 16, isolated as a yellow oil. The crude material is treated with 2-hydroxypyridine (2-HP) to cyclize it to 17. This ester is hydrolyzed to give acid 18. Conversion to 13 is carried out by adding ClCO2Et, followed by reaction with liquid NH3 in the presence of K2CO3. The overall yield of 13 is 32%.
This route is also used to prepare levetiracetam (1) by treating 5 with the HCl salt of amino ester 19 to give 20, recovered as its HCl salt in 49% yield. The salt is basified with Et3N and treated with 2-HP to cyclize it to 1, initially isolated as an oil. GC analysis showed 100% conversion, and chiral HPLC showed that the product contains 98.6% (S)-isomer and 1.4% (R)-isomer.
The inventors also prepared 1 and its (R)-enantiomer 21 by using a similar reaction scheme with alternative substrates to 5. Figure 4 outlines the route, which starts from protected hydroxy amide 22 and amino ester 23. When the reaction is carried out in the presence of Cs2CO3, the product is (R)-enantiomer24, which is used without purification to prepare 21 by treating it with 2-HP. Chiral HPLC showed that the product is 94% (R) and 6% (S).
When the reaction between 22 and 23 is run with K2CO3, the product is (S)-enantiomer 25. This is used to prepare 1, but the product contains only 79% (S)-isomer.
The inventors do not comment on the apparent stereoselectivity of the carbonate salts in the reaction of 22 with 23. This is an intriguing finding and worthy of investigation. (UCB S.A. [Brussels]. US Patent 8,338,621
SYNTHESIS
PATENT
WO2005028435
Example 1: Synthesis of (2S)-2-((4R)-2-oxo-4-n-propyl-l-pyrrolidinyl)butanamide 1.1 Synthesis of (2S)-2-aminobutyramide free base
1800 ml of isopropanol are introduced in a 5L reactor. 1800 g of (2S)-2- aminobutyramide tartrate are added under stirring at room temperature. 700 ml of a 25% aqueous solution of ammonium hydroxide are slowly added while maintaining the temperature below 25°C. The mixture is stirred for an additional 3 hours and then the reaction is allowed to complete at 18°C for 1 hour. The ammonium tartrate is filtered. Yield : 86%.
1.2 Synthesis of 5-hydroxy-4-n-propyl-furan-2-one
Heptane (394 ml) and morpholine (127.5 ml) are introduced in a reactor. The mixture is cooled to 0°C and glyoxylic acid (195 g, 150 ml, 50w% in water) is added. The mixture is heated at 20°C during 1 hour, and then valeraldehyde (148.8 ml) is added . The reaction mixture is heated at 43°C during 20 hours. After cooling down to 20CC, a 37 % aqueous solution of HCl (196.9 ml) is slowly added to the mixture, which is then stirred during 2 hours.
After removal of the heptane phase, the aqueous phase is washed three times with heptane. Diisopropyl ether is added to the aqueous phase. The organic phase is removed, and the aqueous phase further extracted with diisopropyl ether (2x). The diisopropyl ether phases are combined, washed with brine and then dried by azeotropic distillation. After filtration and evaporation of the solvent, 170g of 5- hydroxy-4-n-propyl-furan-2-one are obtained as a brown oil. Yield: 90.8 %
1.3 Synthesis of (2S)-2-((4R)-2-oxo-4-n-propyl-l-pyrrolidinyl)butanamide and (2S)-2-((4S)-2-oxo-4-n-propyl-l-pyrrolidinyl)butanamide
(S, R) (S, S) The (2S)-2-aιninobutyrarnide solution in isopropanol containing 250 g obtained as described here above is dried by azeotropic distillation under vacuum. To the dried (2S)-2-am obutyraιnide solution is added 5-hydroxy-4-n-propyl-furan-2-one (290 g) between 15°C and 25 °C; the mixture is heated to 30 °C and kept for at least 2 hours at that temperature. Acetic acid (1, 18 eq.), Pd/C catalyst (5 w/w%; Johnson Matthey 5% Pd on carbon – type 87L) are then added and hydrogen introduced into the system under pressure. The temperature is kept at 40 °C maximum and the H2 pressure maintained between 0,2 bar and 0,5 bar followed by stirring for at least 20 hours following the initial reaction. The solution is then cooled to between 15 °C and 25 °C and filtered to remove the catalyst. The solution of product in isopropanol is solvent switched to a solution of product in isopropyl acetate by azeotropic distillation with isopropyl acetate. The organic solution is washed with aqueous sodium bicarbonate followed by a brine wash and then filtered. After recristallisation, 349 g of (2S)-2-((4R)-2- oxo-4-n-propyl-l-pyrrolidinyl)butanamide and (2S)-2-((4S)-2-oxo-4-n-propyl-l- pyιτolidinyl)butanamide are obtained (Yield: 82.5%).
1.4 Preparation of (2S)-2-((4R)-2-oxo-4-n-propyl-l-pyrrolidinyl)butanamide The chromatographic separation of the two diastereoisomers obtained in 1.3 is performed using of (CHIRALPAK AD 20 um) chiral stationary phase and a 45/55 (volume /volume) mixture of n-heptane and ethanol as eluent at a temperature of 25 + 2°C. The crude (2S)-2-((4R)-2-oxo-4-n-propyl-l-pyrrolidinyl)butanamide thus obtained is recristallised in isopropylacetate, yielding pure (2S)-2-((4R)-2-oxo-4-n-propyl-l- pyrrolidinyl)butanamide (Overall yield: 80%) .
Example 2: Synthesis of (2S)-2-((4R)-2-oxo-4-n-propyl-l-pyrrolidinyl)butanamide
Example 1 is repeated except that in step 1.1 a solution of (2S)-2- aminoburyramide.HCl in isopropanol is used (27.72 g, 1.2 equivalent), which is neutralised with a NHs/isopropanol solution (3,4-3,7 mol/L). The resulting ainmonium chloride is removed from this solution by filtration and the solution is directly used for reaction -with 5-hydroxy-4-n-propyl-furan-2-one (23.62 g, 1.0 equivalent) without intermediate drying of the (2S)-2-aminobutyramide solution. Yield after separation of the two diastereoisomers and recristallisation: approximately 84%.
1. WO0162726A2.
2. WO2005028435A1 / US2007100150A1.
3. J. Med. Chem. 1988, 31, 893-897.
4. J. Org. Chem. 1981, 46, 4889-4894.
PATENT
Example 3-Synthesis of brivaracetam (I)
3.a. Synthesis of (S) and (R) 2-((R)-2-oxo-4-propyl-pyrrolidin-l-yl)-butyric acid methyl ester fVIaa*) and (Wlab)
(VIaa) (VIab) A slurry of 60% sodium hydride suspension in mineral oil (0.94g, 23.4 mmol) in tetrahydrofuran (30 mL) is cooled at 0°C under a nitrogen atmosphere. A solution of substantially optically pure (R)-4-propyl-pyrrolidin-2-one (Ilia) (2g, 15.7 mmol) in tetrahydrofuran (2 mL) is added over a 15 minutes period. The reaction mixture is stirred 10 min at 0°C then a solution of methyl-2-bromo-butyric acid methyl ester (V) (3.69g, 20.4 mmol) in tetrahydrofuran (2mL) was added over a 20 minutes period. The reaction mixture is stirred at O0C until maximum conversion of starting material and the reaction mixture is then allowed to warm to room temperature and diluted with water (20 mL). Tetrahydrofuran is removed by evaporation and the residue is extracted with isopropyl acetate (20 ml + 10 mL). The combined organic layers are dried on anhydrous magnesium sulfate and evaporated to afford 3g (13.2 mmol, 86 %) of a mixture of epimers of compound (Via), as a mixture respectively of epimer (VIaa) and epimer (VIab). 1H NMR(400 MHz, CDCI3) of the mixture of epimers (VIaa) and (VIab) : δ = 4.68
(dd, J= 10.8, J= 5.1, 2×1 H) ; 3.71 (s, 2x3H); 3.60 (t app, J= 8.2, IH); 3.42 (t app, J= 8.7, IH); 313 (dd, J= 9.2, J = 6.8, IH); 2.95 (dd, J= 9.2, J= 6.8, IH); 2.56 (dd, J= 16.6, J = 8.7, 2xlH); 2.37 (dm, 2xlH); 2.10 (m, 2xlH); 2.00 (m, 2xlH); 1.68 (m, 2xlH); 1.46 (m, 2x2H); 1.36 (m, 2x2H); 0.92 (m, 2x6H).
13C NMR (400 MHz, CDCl3) of the mixture of epimers (VIaa) and (VIab) : δ =
175.9; 175.2; 171.9; 55.3; 52.4; 49.8; 49.5; 38.0; 37.8; 37.3; 36.9; 32.5; 32.2; 22.6; 22.4; 21.0; 14.4; 11.2; 11.1
HPLC (GRAD 90/10) of the mixture of epimers (VIaa) and (VIab): retention time= 9.84 minutes (100 %)
GC of the mixture of epimers (VIaa) and (VIab): retention time = 13.33 minutes (98.9 %)
MS of the mixture of epimers (VIaa) and (VIab) (ESI) : 228 MH+
3.b. Ammonolysis of compound of the mixture of (VIaa) and (VIab)
(VIaa) (VIab) (I) (VII)
A solution of (VIaa) and (VIab) obtained in previous reaction step (1.46g, 6.4 mmol) in aqueous ammonia 50 % w/w (18 mL) at 00C is stirred at room temperature for 5.5hours. A white precipitate that appears during the reaction, is filtered off, is washed with water and is dried to give 0.77g (3.6 mmol, yield = 56 %) of white solid which is a mixture of brivaracetam (I) and of compound (VII) in a 1 :1 ratio.
1H NMR of the mixture (I) and (VII) (400 MHz, CDCI3) : δ = 6.36 (s, broad, IH); 5.66 (s, broad, IH); 4.45 (m, IH); 3.53 (ddd, J= 28.8, J= 9.7, J= 8.1, IH); 3.02 (m, IH); 2.55 (m, IH); 2.35 (m, IH); 2.11 (m, IH); 1.96 (m, IH); 1.68 (m, IH); 1.38 (dm, 4H); 0.92 (m, 6H). 13c NMR of the mixture (I) and (VII) (400 MHz, CDCl3) : δ = 176.0; 175.9; 172.8;
172.5; 56.4; 56.3; 50.0; 49.9; 38.3; 38.1; 37.3; 37.0; 32.3; 32.2; 21.4; 21.3; 21.0; 20.9; 14.4; 10.9; 10.8
HPLC (GRAD 90/10) of the mixture of (I) and (VII) retention time= 7.67 minutes (100 %)
Melting point of the mixture of (I) and (VII) = 104.90C (heat from 400C to 1200C at 10°C/min)
Compounds (I) and (VII) are separated according to conventional techniques known to the skilled person in the art. A typical preparative separation is performed on a 11.7g scale of a 1 :1 mixture of compounds (I) and (VII) : DAICEL CHIRALPAK® AD 20 μm, 100*500 mm column at 300C with a 300 mL/minutes debit, 50 % EtOH – 50 % Heptane. The separation affords 5.28g (45 %) of compound (VII), retention time = 14 minutes and 5.2Og (44 %) of compounds (I), retention time = 23 minutes.
1H NMR of compound (I) (400 MHz, CDCl3): δ = 6.17 (s, broad, IH); 5.32 (s, broad, IH); 4.43 (dd, J= 8.6, J= 7.1, IH); 3.49 (dd, J= 9.8, J= 8.1, IH); 3.01 (dd, J= 9.8, J= 7.1, IH); 2.59 (dd, J= 16.8, J= 8.7, IH); 2.34 (m, IH); 2.08 (dd, J= 16.8, J= 7.9, IH); 1.95 (m, IH); 1.70 (m, IH); 1.47-1.28 (m, 4H); 0.91 (dt, J= 7.2, J= 2.1, 6H)
HPLC (GRAD 90/10) of compound (I) : retention time = 7.78 minutes
1H NMR of compound (VII) (400 MHz, CDCl3): δ = 6.14 (s, broad, IH); 5.27 (s, broad, IH); 4.43 (t app, J = 8.1, IH); 3.53 (t app, J = 9.1, IH); 3.01 (t app, J = 7.8, IH); 2.53 (dd, J = 16.5, J = 8.8, IH); 2.36 (m, IH); 2.14 (dd, J = 16.5, J = 8.1, IH); 1.97 (m, IH); 1.68 (m, IH); 1.43 (m, 2H); 1.34 (m, 2H); 0.92 (m, 6H)
3c. Epimerisation of compound of (2RV2-((R)-2-oxo-4-propyl-pyπOlidin-l-ylV butyramide (VID
Compound (VII) (200 mg, 0.94 mmol) is added to a solution of sodium tert- butoxide (20 mg, 10 % w/w) in isopropanol (2 mL) at room temperature. The reaction mixture is stirred at room temperature for 18h. The solvent is evaporated to afford 200 mg
(0.94 mmol, 100 %) of a white solid. Said white solid is a mixture of brivaracetam (I) and of (VII) in a ratio 49.3 / 50.7.
HPLC (ISO80): retention time= 7.45 min (49.3%) brivaracetam (I); retention time= 8.02 minutes (50.7%) compound (VII).
1. WO2007031263A1 / US2009318708A1.
PATENT
(scheme 3).
Scheme 3
scheme 4.
5h. Synthesis of brivaracetam and (V) A suspension of (Id) and (Ie) (0.6 g, 2.3 mmol) in MIBK (10 mL) is heated at
120°C for 6 hours. The resulting solution is concentrated and separated on chromatography column (Silicagel 600.068-0.200 mm, cyclohexane/EtOAc : 10/90) to give 0.13 g of brivaracetam (0.6 mmol, 26 %, ee = 94 %) and (V).
1H NMR (400 MHz, CDCl3): δ = 6.17 (s, broad, IH); 5.32 (s, broad, IH); 4.43 (dd, J= 8.6, J= 7.1, IH); 3.49 (dd, J= 9.8, J= 8.1, IH); 3.01 (dd, J= 9.8, J= 7.1, IH); 2.59 (dd, J= 16.8, J= 8.7, IH); 2.34 (m, IH); 2.08 (dd, J= 16.8, J= 7.9, IH); 1.95 (m, IH); 1.70 (m, IH); 1.47-1.28 (m, 4H); 0.91 (dt, J= 7.2,J= 2.1, 6H).
HPLC (method 90/10) : Retention time = 7.78 minutes Chiral HPLC : Retention time = 9.66 minutes (97%) MS (ESI): 213 MH+
1. WO2007065634A1 / US2009012313A1.
References
- von Rosenstiel P (Jan 2007). “Brivaracetam (UCB 34714)”. Neurotherapeutics 4 (1): 84–7.doi:10.1016/j.nurt.2006.11.004.PMID 17199019.
- Malawska B, Kulig K (Jul 2005). “Brivaracetam UCB”. Current Opinion in Investigational Drugs 6 (7): 740–746. PMID 16044671.
- Rogawski MA, Bazil CW (Jul 2008). “New molecular targets for antiepileptic drugs: alpha(2)delta, SV2A, and K(v)7/KCNQ/M potassium channels”. Current Neurology and Neuroscience Reports 8 (4): 345–352. doi:10.1007/s11910-008-0053-7.PMC 2587091.PMID 18590620.
- Clinical trial number NCT00464269 for “Double-blind, Randomized Study Evaluating the Efficacy and Safety of Brivaracetam in Adults With Partial Onset Seizures” at ClinicalTrials.gov
- Rogawski MA (Aug 2008). “Brivaracetam: a rational drug discovery success story”. British Journal of Pharmacology 154 (8): 1555–7.doi:10.1038/bjp.2008.221. PMC 2518467. PMID 18552880.
- Matagne A, Margineanu DG, Kenda B, Michel P, Klitgaard H (Aug 2008). “Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A”. British Journal of Pharmacology 154 (8): 1662.doi:10.1038/bjp.2008.198. PMID 18500360.
- http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003898/human_med_001945.jsp&mid=WC0b01ac058001d124
- http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm486827.htm
| Names | |||
|---|---|---|---|
| IUPAC name
(2S)-2-[(4R)-2-oxo- 4-propylpyrrolidin-1-yl] butanamide
| |||
| Identifiers | |||
| 357336-20-0 | |||
| ChEMBL | ChEMBL607400 | ||
| ChemSpider | 8012964 | ||
| Jmol interactive 3D | Image | ||
| PubChem | 9837243 | ||
| UNII | U863JGG2IA | ||
| Properties | |||
| C11H20N2O2 | |||
| Molar mass | 212.15 g/mol | ||
| Pharmacology | |||
| ATC code | N03 | ||
| Legal status |
| ||
| Oral | |||
| Pharmacokinetics: | |||
| Nearly 100% | |||
| <20% | |||
| Hydrolysis, CYP2C8-mediated hydroxylation | |||
| 8 hrs | |||
| >75% renal | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
//////BRIVARACETAM, UCB, 2016 FDA, UCB-34714
CCCC1CC(=O)N(C1)C(CC)C(=O)N


