 .
.Picture credit....Bethany Halford
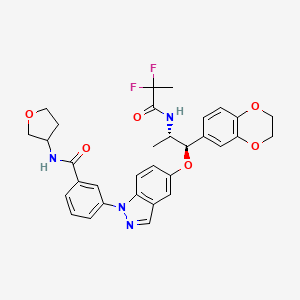
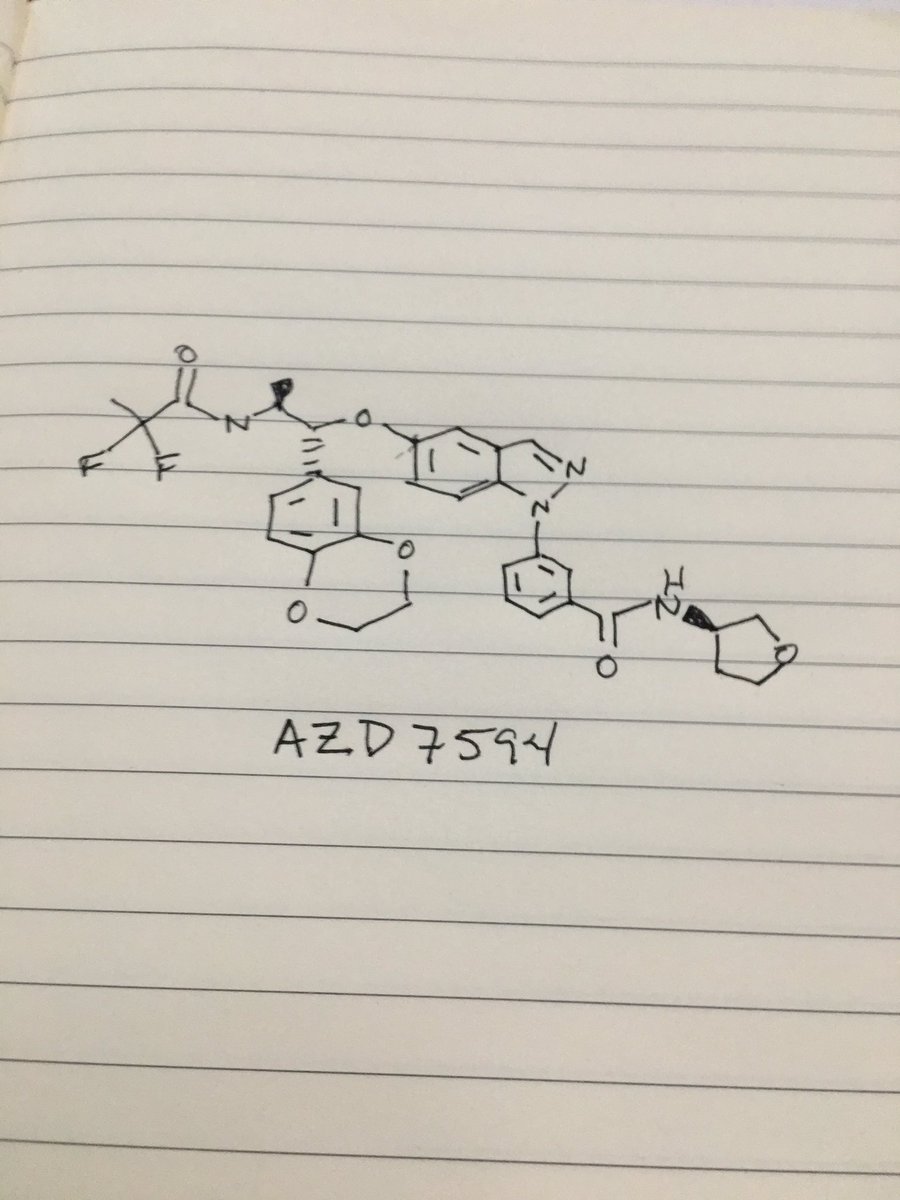
AZD 7594
AZ13189620; AZD-7594
Bayer Pharma Aktiengesellschaft, Astrazeneca Ab
| Molecular Formula: | C32H32F2N4O6 |
|---|---|
| Molecular Weight: | 606.616486 g/mol |
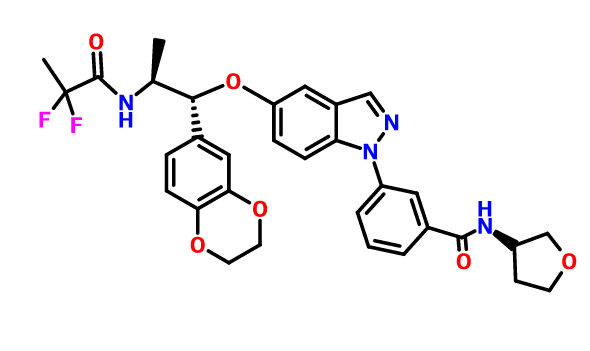
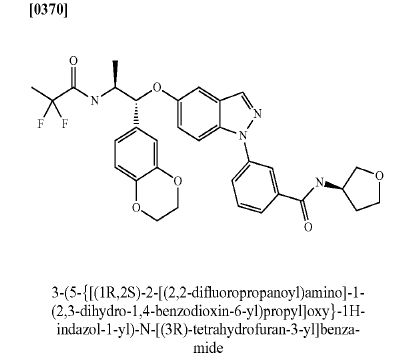
Benzamide, 3-[5-[(1R,2S)-2-[(2,2-difluoro-1-oxopropyl)amino]-1-(2,3-dihydro-1,4-benzodioxin-6-yl)propoxy]-1H-indazol-1-yl]-N-[(3R)-tetrahydro-3-furanyl]-
- Cas 1196509-60-0
It is also in phase I clinical trials for the treatment of chronic obstructive pulmonary disorder (COPD).
https://clinicaltrials.gov/ct2/show/NCT02479412
| Company | AstraZeneca plc |
| Description | Inhaled selective glucocorticoid receptor (GCCR) modulator |
| Molecular Target | Glucocorticoid receptor (GCCR) |
- Phase II Asthma
- Phase I Chronic obstructive pulmonary disease
- 01 Feb 2016 AstraZeneca completes a phase II trial in Asthma in Bulgaria and Germany (Inhalation) (NCT02479412)
- 09 Jan 2016 AstraZeneca plans to initiate a phase I trial in Healthy volunteers in USA (IV and PO) (NCT02648438)
- 01 Jan 2016 Phase-I clinical trials in Chronic obstructive pulmonary disease (In volunteers) in USA (PO, IV, Inhalation) (NCT02648438)
http://www.google.com/patents/WO2009142569A1
PATENT
US20100804345
UNWANTED ISOMER
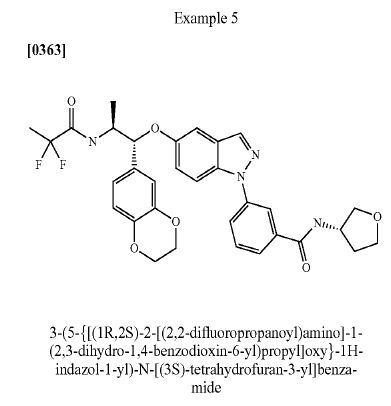
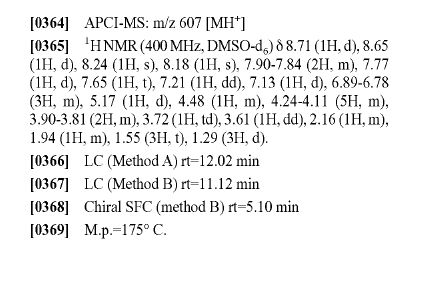
WANTED COMPD



PATENT
WO 2009142571Example 6
WANTED ISOMER
3-(5- { TC 1 R,2SV2-r(2,2-difluoropropanoyl)aminol- 1 -(2,3-dihydro-l ,4-benzodioxin-6-5 yDpropylioxy) - 1 H-indazol- 1 -ylVN-[(3R)-tetrahydrofuran-3-vnbenzamide. APCI-MS: m/z 607 [MH+] 1H NMR ^OO MHz, DMSOd6) δ 8.71 (IH, d), 8.65 (IH, d), 8.24 (IH, s), 8.18 (IH, s), 7.90 - 7.84 (2H, m), 7.77 (IH, d), 7.65 (IH, t), 7.21 (IH, dd), 7.13 (IH, d), 6.89 - 6.78 (3H, m), 5.17 (IH, d), 4.48 (IH, m), 4.23 - 4.10 (5H, m), 3.89 - 3.82 (2H, m), 3.72 (IH, td), 3.61 (IH, dd), 2.16 (IH, m), 1.94 (IH, m), 1.55 (3H, t), 1.29 (3H, d). LC (method A) rt = 12.03 min LC (method B) rt = 11.13 min Chiral SFC (method B) rt = 4.71 min M.p. = 177 °C
UNWANTED
o 3-(5- { IY 1 R,2S V2-r(2,2-difluoropropanoyl')amino'|- 1 -(2,3-dihydro- 1 ,4-benzodioxin-6- yl)propyl]oxy } - 1 H-indazol- 1 -yP-N-IO S)-tetrahydrofuran-3 -yl"|benzamide
APCI-MS: m/z 607 [MH+]
1H NMR (400 MHz, DMSO-J6) δ 8.71 (IH, d), 8.65 (IH, d), 8.24 (IH, s), 8.18 (IH, s),
7.90 - 7.84 (2H, m), 7.77 (IH, d), 7.65 (IH, t), 7.21 (IH, dd), 7.13 (IH, d), 6.89 - 6.78 (3H,s m), 5.17 (IH, d), 4.48 (IH, m), 4.24 - 4.11 (5H, m), 3.90 - 3.81 (2H, m), 3.72 (IH, td), 3.61
(IH, dd), 2.16 (IH, m), 1.94 (IH, m), 1.55 (3H, t), 1.29 (3H, d).
LC (Method A) rt = 12.02 min
LC (Method B) rt = 11.12 min
Chiral SFC (method B) rt = 5.10 min o M.p. = 175 0C
PATENT
WO 2011061527http://www.google.com/patents/WO2011061527A1?cl=en
Intermediate 12
( 1 R,2S)-2-amino- 1 -(2,3 -dihydrobenzo b [ 1 ,41dioxin-6-yl)propan- 1 -ol hydrochloride. (12)
5-6 N HC1 in 2-propanol (8 mL, 40-48 mmol) was added to tert-butyl (lR,2S)-l-(2,3- dihydrobenzo[b][l,4]dioxin-6-yl)-l-hydroxypropan-2-ylcarbamate (I2a) (3.1 g, 10.02 mmol) in ethyl acetate (40 mL) at 40°C and stirred for 3 hours. The reaction mixture was allowed to reach r.t. and was concentrated by evaporation. Ether was added and the salt was filtered off and washed with ether. The salt was found to be hygroscopic. Yield 2.10 g (85%)
APCI-MS: m/z 210 [MH+-HC1]
1H-NMR (300 MHz, DMSO-^): δ 8.01 (brs, 3H), 6.87-6.76 (m, 3H), 5.93 (brd, 1H), 4.79 (brt, 1H), 4.22 (s, 4H), 3.32 (brm, 1H), 0.94 (d, 3H).
tert-butyl (1R,2S)- 1 -(2,3-dihvdrobenzorbl Γ 1 ,41dioxin-6-yl)- 1 -hvdroxypropan-2-ylcarbamate.
The diastereoselective catalytic Meerwein-Ponndorf-Verley reduction was made by the method described by Jingjun Yin et. al. J. Org. Chem. 2006, 71, 840-843.
(S)-tert-butyl 1 -(2,3-dihydrobenzo[b] [ 1 ,4]dioxin-6-yl)- 1 -oxopropan-2-ylcarbamate (I2b) (3.76 g, 12.23 mmol), aluminium isopropoxide (0.5 g, 2.45 mmol) and 2-propanol (12 mL, 157.75 mmol) in toluene (22 mL) were stirred at 50°C under argon for 16 hours. The reaction mixture was poured into 1M HC1 (150 mL) and the mixture was extracted with ethyl acetate (250 mL). The organic phase was washed with water (2x50 mL) and brine (100 mL), dried over Na2SC"4, filtered and concentrated. The crude product was purified by flash- chromatography on silica using ethyl acetate/hexane (1/2) as eluent. Fractions containing product were combined. Solvent was removed by evaporation to give the desired product as a colourless solid. Yield 3.19 g (84%) APCI-MS: m/z 236, 210, 192 [MH -tBu-18, MH -BOC, MH -BOC- 18]
1H NMR (300 MHz, DMSO-^): δ 6.80-6.70 (m, 3H), 6.51 (d, IH), 5.17 (d, IH), 4.36 (t, IH),
4.19 (s, 4H), 3.49 (m, IH), 1.31 (s, 9H), 0.93 (d, 3H).
(S)-tert-butyl 1 -(2,3-dihydrobenzo[bl [ 1 ,41dioxin-6-yD- 1 -oxopropan-2-ylcarbamate. (I2b)
A suspension of (S)-tert-butyl l-(methoxy(methyl)amino)-l-oxopropan-2-ylcarbamate (3 g, 12.92 mmol) in THF (30 mL) was placed under a protective atmosphere of argon and cooled down to -15 to -20°C. Isopropylmagnesium chloride, 2M in THF (6.5 mL, 13.00 mmol), was added keeping the temperature below -10°C. The temperature was allowed to reach 0°C. A freshly prepared solution of (2,3-dihydrobenzo[b][l,4]dioxin-6-yl)magnesium bromide, 0.7M in THF (20 mL, 14.00 mmol) was added. The temperature was allowed to reach r.t. overnight. The reaction mixture was poured into ice cooled IN HC1 (300 mL). TBME (300 mL) was added and the mixture was transferred to a separation funnel. The water phase was back extracted with TBME (200 mL). The ether phases were washed with water, brine and dried (Na2S04). The crude product was purified by flash chromatography using TBME /Heptane 1/2 as eluent. Fractions containing the product were combined and solvents were removed by evaporation to give the subtitle compound as a slightly yellow sticky oil/gum. Yield 3.76g
(95%)
APCI-MS: m/z 208 [MH+ - BOC]
1H NMR (300 MHz, DMSO-^): δ 7.50 (dd, IH), 7.46 (d, IH), 7.24 (d, IH), 6.97 (d, IH), 4.97 (m, IH), 4.30 (m, 4H), 1.36 (s, 9H), 1.19 (d, 3H).
Intermediate 13
(lR,2S)-2-amino-l-(4H-benzo[dl[l,31dioxin-7- l)propan-l-ol hydrochloride (13)
Tert-butyl ( 1 R,2S)- 1 -(4H-benzo[d] [ 1 ,3]dioxin-7-yl)- 1 -hydroxypropan-2-ylcarbamate (I3b) (403 mg, 1.30 mmol) was dissolved in ethyl acetate (5 mL) and 5-6 N HC1 solution in 2- propanol (1.5 mL, 7.5-9 mmol) was added. The mixture was stirred at 50 °C for 1.5 hours. The solvents was removed by evaporation. The residual sticky gum was treated with ethyl acetate and evaporated again to give a solid material that was suspended in acetonitrile and stirred for a few minutes. The solid colourless salt was collected by filtration and was found to be somewhat hygroscopic. The salt was quickly transferred to a dessicator and dried under reduced pressure. Yield 293 mg (92%)
APCI-MS: m/z 210 [MH+ -HC1]
1H NMR (300 MHz, DMSO-^) δ 8.07 (3H, s), 7.05 (IH, d), 6.92 (IH, dd), 6.85 (IH, d), 6.03 (IH, d), 5.25 (2H, s), 4.87 (3H, m), 3.42 - 3.29 (IH, m), 0.94 (3H, d).
(4S.5R -5-(4H-benzordiri.31dioxin-7-vn- -methyloxazolidin-2-one (I3a
A mixture of (lR,2S)-2-amino-l-(4H-benzo[d][l,3]dioxin-7-yl)propan-l-ol hydrochloride (I3b) (120 mg, 0.49 mmol), DIEA (0.100 mL, 0.59 mmol) and CDI (90 mg, 0.56 mmol) in THF (2 mL) was stirred at r.t. for 2 hours. The reaction mixture was concentrated by evaporation and the residual material was partitioned between ethyl acetate and water. The organic phase was washed with 10% NaHS04, dried over MgS04, filtered and evaporated. The crude product was analysed by LC/MS and was considered pure enough for further analysis by NMR. Yield 66 mg (57%)
The relative cis conformation of the product was confirmed by comparing the observed 1H- NMR with the literature values reported for similar cyclised norephedrine (Org. Lett. 2005 (07), 13, 2755-2758 and Terahedron Assym. 1993, (4), 12, 2513-2516). In a 2D NOESY experiment a strong NOE cross-peak was observed for the doublet at 5.64 with the multiplet at 4.19 ppm. This also confirmed the relative czs-conformation.
APCI-MS: m/z 236 [MH+]
1H NMR (400 MHz, CDC13) δ 6.99 (d, J= 8.0 Hz, IH), 6.88 (dd, J= 8.0, 1.4 Hz, IH), 6.83 (s, IH), 5.81 (brs,lH), 5.64 (d, J= 8.0 Hz, IH), 5.26 (s, 2H), 4.91 (s, 2H), 4.19 (m, IH), 0.85 (d, J = 6.4 Hz, 3H). Tert-butyl ( 1 R,2S)- 1 -(4H-benzord1 Γ 1 ,31dioxin-7-yl)- 1 -hvdroxypropan-2-ylcarbamate (I3b)
A mixture (S)-tert-butyl l-(4H-benzo[d][l,3]dioxin-7-yl)-l-oxopropan-2-ylcarbamate (I3c) (680 mg, 2.21 mmol), triisopropoxyaluminum (140 mg, 0.69 mmol) and propan-2-ol (3 mL, 38.9 mmol) in toluene (3 mL) was stirred at 65 °C for 15 hours. The reaction mixture was allowed to cool down, poured into 1M HC1 (50 mL) and extracted with ethyl acetate (2x50 mL). The organic phase was washed with water, brine, dried over MgS04, filtered and solvents were removed by evaporation to afford a colourless solid. The crude product was purified by flash chromatography, (solvent A = Heptane, solvent B = EtOAc + 10% MeOH. A gradient of 10%B to 50%B in A was used). The obtained product was crystallised from DCM / heptane to afford the subtitle compound as colourless needles. Yield 414 mg (60%)
APCI-MS: m/z 210 [MH+ -BOC]
1H NMR (400 MHz, DMSO- ¾ δ 6.97 (1H, d), 6.88 (1H, d), 6.77 (1H, s), 6.56 (1H, d), 5.27 (1H, d), 5.22 (2H, s), 4.83 (2H, s), 4.44 (1H, t), 3.53 (1H, m), 1.32 (9H, s), 0.93 (3H, d). (S)-Tert-butyl 1 -(4H-benzord1 Γ 1 ,31dioxin-7-vD- 1 -oxopropan-2-ylcarbamate (I3c)
7-Bromo-4H-benzo[d][l,3]dioxine (1 g, 4.65 mmol) was dissolved in THF (5 mL) and added to magnesium (0.113 g, 4.65 mmol) under a protective atmosphere of argon. One small iodine crystal was added. The coloured solution was heated with an heat gun in short periods to initiate the Grignard formation. When the iodine colour vanished the reaction was allowed to proceed at r.t. for 1.5 hours.
In a separate reaction tube (S)-tert-butyl l-(methoxy(methyl)amino)-l-oxopropan-2- ylcarbamate (1 g, 4.31 mmol) was suspended in THF (5 mL) and cooled in an ice/acetone bath to below -5 °C. Isopropylmagnesium chloride, 2M solution in THF (2.5 mL, 5.00 mmol) was slowly added to form a solution. To this solution was added the above freshly prepared Grignard reagent. The mixture was allowed to reach r.t. and stirred for 4 hours. The reaction mixture was slowly poured into ice-cold 150 mL 1M HC1. Ethyl acetate (150 mL) was added and the mixture was stirred for a few minutes and transferred to a separation funnel. The organic phase was washed with water and brine, dried over MgS04, filtered and concentrated. The obtained crude product was further purified by flash chromatography using a prepacked 70g silica column with a gradient of 10% TBME to 40% TBME in heptane as eluent. The subtitle compound was obtained as a colourless solid. Yield 790 mg (59%>)
APCI-MS: m/z 208 [MH+ -BOC]
1H NMR (400 MHz, DMSO-^) δ 7.53 (IH, dd), 7.39 (IH, s), 7.30 (IH, d), 7.22 (IH, d), 5.30 (2H, s), 4.98 (IH, m), 4.95 (2H, s), 1.35 (9H, s), 1.20 (3H, d).
Preparation 4
3-(5-([(lR,2S)-2-[(2,2-difluoropropanoyl)aminol-l-(2,3-dihydro-l,4-benzodioxin-6- yl)propyl]oxy| - 1 H-indazol- 1 -yl)-N-[(3R)-tetrahydrofuran-3-yllbenzamide
TEA (2.0 g, 20.65 mmol) was added to a mixture of 3-(5-((lR,2S)-2-(2,2- difluoropropanamido)- 1 -(2,3-dihydrobenzo[b] [ 1 ,4]dioxin-6-yl)propoxy)-l H-indazol-1 - yl)benzoic acid (14) (3.6 g, 6.70 mmol), (R)-tetrahydrofuran-3 -amine hydrochloride (0.99 g, 8.0 mmol) and HBTU (2.65 g, 6.99 mmol) in DCM (15 mL). The reaction was stirred at r.t. for 3h, then quenched by addition of a mixture of water and ethyl acetate. The mixture was shaken and the organic layer was collected. The water phase was extracted twice with ethyl acetate. The combined organic layers were washed with a small portion of water and dried over magnesium sulphate. The product was purified by flash chromatography (silica, eluent: a gradient of ethyl acetate in heptane). The residue was crystallized by dissolving in refluxing acetonitrile (50 mL) and then allowing to cool to r.t. over night. The solid was collected by filtration, washed with a small volume of acetonitrile and dried at 40°C in vaccum to give the title compound (2.5 g, 61%).
APCI-MS: m/z 607 [MH+]
1H NMR (400 MHz, DMSO-d6) δ 8.71 (IH, d), 8.65 (IH, d), 8.24 (IH, s), 8.18 (IH, s), 7.90 - 7.84 (2H, m), 7.77 (IH, d), 7.65 (IH, t), 7.21 (IH, dd), 7.13 (IH, d), 6.89 - 6.78 (3H, m), 5.17 (IH, d), 4.48 (IH, m), 4.23 - 4.10 (5H, m), 3.89 - 3.82 (2H, m), 3.72 (IH, td), 3.61 (IH, dd), 2.16 (IH, m), 1.94 (IH, m), 1.55 (3H, t), 1.29 (3H, d).
LC (method A) rt = 12.03 min
LC (method B) rt = 11.13 min
Chiral SFC (method B) rt = 4.71 min
M.p. = 177 °C
| Patent ID | Date | Patent Title |
|---|---|---|
| US2015080434 | 2015-03-19 | PHENYL AND BENZODIOXINYL SUBSTITUTED INDAZOLES DERIVATIVES |
| US8916600 | 2014-12-23 | Phenyl and benzodioxinyl substituted indazoles derivatives |
| US8211930 | 2012-07-03 | Phenyl and Benzodioxinyl Substituted Indazoles Derivatives |
https://www.astrazeneca.com/content/dam/az/press-releases/2014/Q2/Pipeline-table.pdf
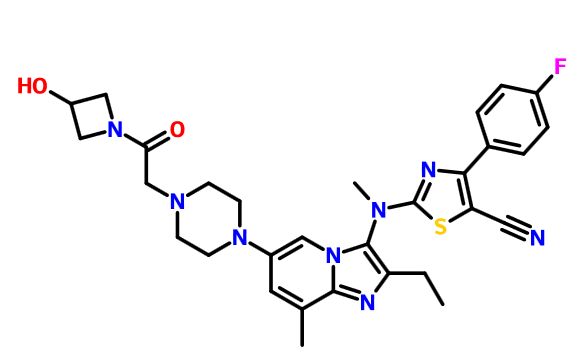
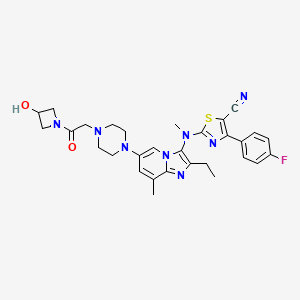
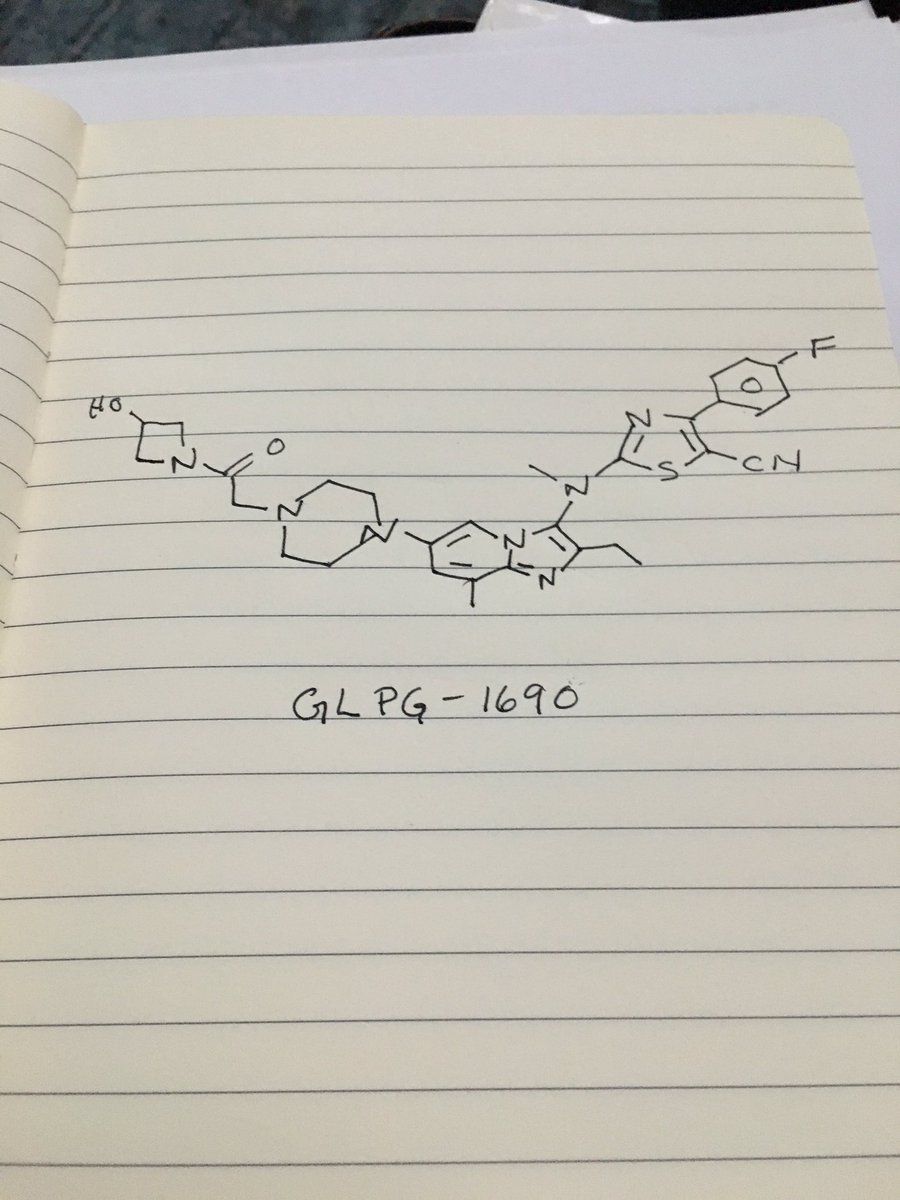
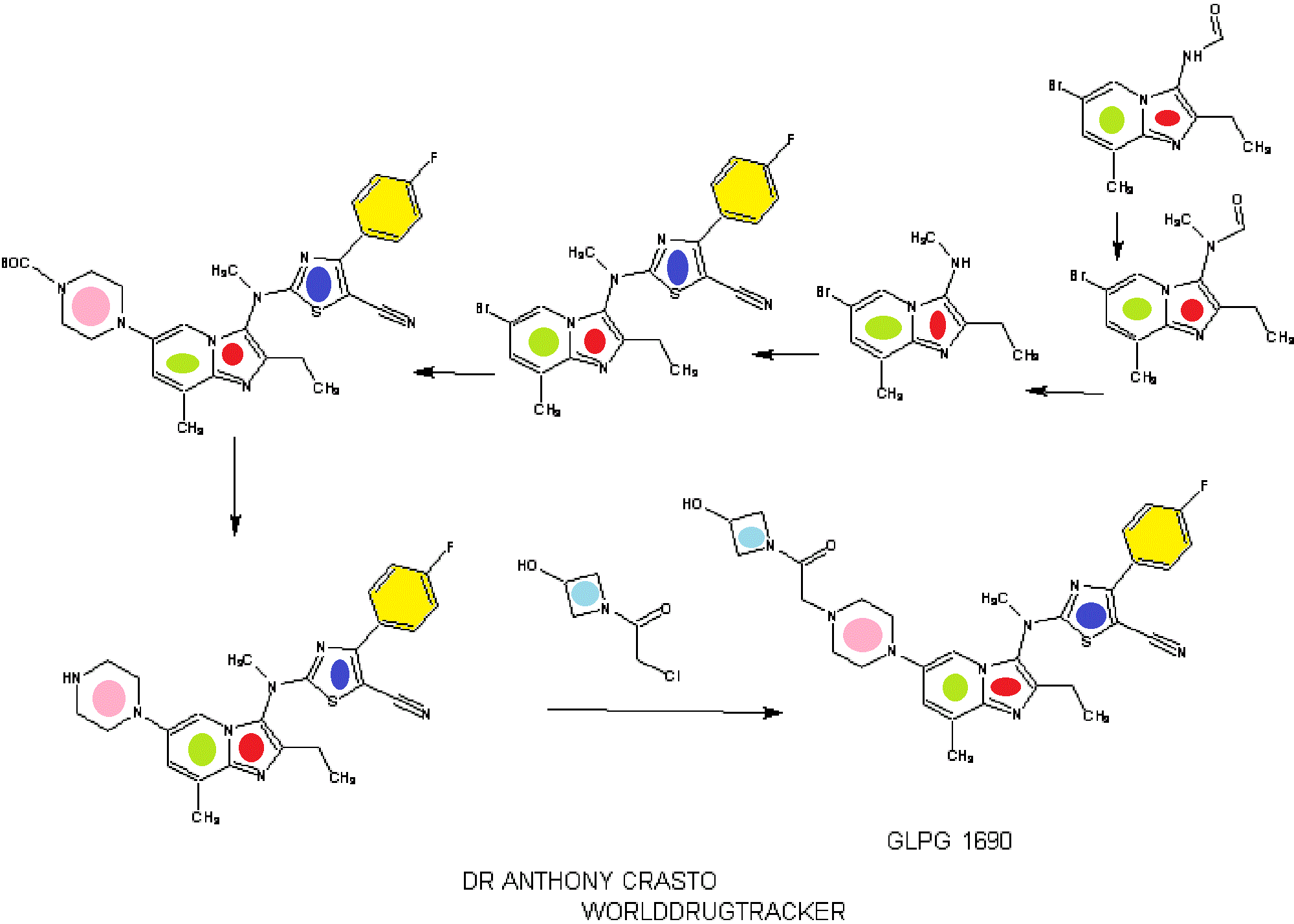
////////AZD 7594, AZ13189620, AZD-7594 , phase 2, astrazeneca, 1196509-60-0
c21cc(ccc1n(nc2)c3cc(ccc3)C(=O)NC4COCC4)O[C@H](c5cc6c(cc5)OCCO6)[C@@H](NC(=O)C(F)(F)C)C
CC(C(C1=CC2=C(C=C1)OCCO2)OC3=CC4=C(C=C3)N(N=C4)C5=CC=CC(=C5)C(=O)NC6CCOC6)NC(=O)C(C)(F)F
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
 amcrasto@gmail.com
amcrasto@gmail.com
P.STHE VIEWS EXPRESSED ARE MY PERSONAL AND IN NO-WAY SUGGEST THE VIEWS OF THE PROFESSIONAL BODY OR THE COMPANY THAT I REPRESENT, amcrasto@gmail.com, +91 9323115463 India.
I , Dr A.M.Crasto is writing this blog to share the knowledge/views, after reading Scientific Journals/Articles/News Articles/Wikipedia. My views/comments are based on the results /conclusions by the authors(researchers). I do mention either the link or reference of the article(s) in my blog and hope those interested can read for details. I am briefly summarising the remarks or conclusions of the authors (researchers). If one believe that their intellectual property right /copyright is infringed by any content on this blog, please contact or leave message at below email address amcrasto@gmail.com. It will be removed ASAP