Dofequidar fumarate
Phase III
A P-glycoprotein inhibitor potentially for the treatment of breast cancer and non-small lung cancer (NSCLC).
MS-209; Dofequidar fumarate
CAS No. 129716-58-1 (Dofequidar FREE )
CAS No 153653-30-6 (Dofequidar fumarate 1;1)…..C34H35N3O7, 597.66
5-[3-[4-(2,2-Diphenylacetyl)piperazin-1-yl]-2-hydroxypropoxy]quinoline sesquifumarate
1-[4-(2,2-Diphenylacetyl)piperazin-1-yl]-3-(quinoliln-5-yloxy)-2-propanol sesquifumarate
1-(Diphenylacetyl)-4-[(2RS)-2-hydroxy-3-(5-quinolyloxy)propyl]piperazine sesquifumarate
1-[4-(2,2-Diphenylacetyl)piperazin-1-yl]-3-(quinoliln-5-yloxy)-2-propanol sesquifumarate
1-(Diphenylacetyl)-4-[(2RS)-2-hydroxy-3-(5-quinolyloxy)propyl]piperazine sesquifumarate
4-(Diphenylacetyl)-a-[(5-quinolinyloxy)methyl]-1-Piperazineethanol (E)-2-butenedioate fumarate (1:1.5), C30 H31 N3 O3 . 3/2 C4 H4 O4
1-Piperazineethanol, 4-(diphenylacetyl)-α-[(5-quinolinyloxy)methyl]-, (E)-2-butenedioate (2:3)
1-Piperazineethanol, 4-(diphenylacetyl)-α-[(5-quinolinyloxy)methyl]-, (E)-2-butenedioate (2:3)
Dofequidar fumarate(MS-209 fumarate), an orally active quinoline compound, has been reported to overcome MDR by inhibiting ABCB1/P-gp, ABCC1/MDR-associated protein 1, or both.
Dofequidar fumarate(MS-209 fumarate), an orally active quinoline compound, has been reported to overcome MDR by inhibitingABCB1/P-gp, ABCC1/MDR-associated protein 1, or both.
IC50 value:
Target: P-gp
in vitro: MS-209 at 3 microM effectively overcame docetaxel resistance in MDR cancer cells, and this concentration was achieved in blood plasma for > 7 h without serious toxicity [1]. MS-209 restored chemosensitivity of SBC-3 / ADM cells to VP-16, ADM, and VCR in a dose-dependent manner in vitro [2]. dofequidar inhibits the efflux of chemotherapeutic drugs and increases the sensitivity to anticancer drugs in CSC-like side population (SP) cells isolated from various cancer cell lines. Dofequidar treatment greatly reduced the cell number in the SP fraction [3]. In 4-1St cells, which are extremely resistant to ADM and VCR, MS-209 at a concentration of 3 microM enhanced the cytotoxicity of ADM and VCR, 88- and 350-fold, respectively [4].
in vivo: Treatment with docetaxel alone at the maximal tolerated dose (MTD) showed an apparent antitumor activity to an intrinsically resistant HCT-15 tumor xenograft, and MS-209 additionally potentiated the antitumor activity of docetaxel. Against a MCF-7/ADM tumor xenograft expressing larger amounts of P-gp, docetaxel alone at the MTD showed no antitumor activity, whereas the MTD of docetaxel combined with MS-209 greatly reduced MCF-7/ADM tumor growth [1]. Intravenous injection with SBC-3 or SBC-3 / ADM cells produced metastatic colonies in the liver, kidneys and lymph nodes in natural killer (NK) cell-depleted severe combined immunodeficiency (SCID) mice, though SBC-3 / ADM cells more rapidly produced metastases than did SBC-3 cells. Treatment with VP-16 and ADM reduced metastasis formation by SBC-3 cells, whereas the same treatment did not affect metastasis by SBC-3 / ADM cells. Although MS-209 alone had no effect on metastasis by SBC-3 or SBC-3 / ADM cells, combined use of MS-209 with VP-16 or ADM resulted in marked inhibition of metastasis formation by SBC-3 / ADM cells to multiple organs [2].
IC50 value:
Target: P-gp
in vitro: MS-209 at 3 microM effectively overcame docetaxel resistance in MDR cancer cells, and this concentration was achieved in blood plasma for > 7 h without serious toxicity [1]. MS-209 restored chemosensitivity of SBC-3 / ADM cells to VP-16, ADM, and VCR in a dose-dependent manner in vitro [2]. dofequidar inhibits the efflux of chemotherapeutic drugs and increases the sensitivity to anticancer drugs in CSC-like side population (SP) cells isolated from various cancer cell lines. Dofequidar treatment greatly reduced the cell number in the SP fraction [3]. In 4-1St cells, which are extremely resistant to ADM and VCR, MS-209 at a concentration of 3 microM enhanced the cytotoxicity of ADM and VCR, 88- and 350-fold, respectively [4].
in vivo: Treatment with docetaxel alone at the maximal tolerated dose (MTD) showed an apparent antitumor activity to an intrinsically resistant HCT-15 tumor xenograft, and MS-209 additionally potentiated the antitumor activity of docetaxel. Against a MCF-7/ADM tumor xenograft expressing larger amounts of P-gp, docetaxel alone at the MTD showed no antitumor activity, whereas the MTD of docetaxel combined with MS-209 greatly reduced MCF-7/ADM tumor growth [1]. Intravenous injection with SBC-3 or SBC-3 / ADM cells produced metastatic colonies in the liver, kidneys and lymph nodes in natural killer (NK) cell-depleted severe combined immunodeficiency (SCID) mice, though SBC-3 / ADM cells more rapidly produced metastases than did SBC-3 cells. Treatment with VP-16 and ADM reduced metastasis formation by SBC-3 cells, whereas the same treatment did not affect metastasis by SBC-3 / ADM cells. Although MS-209 alone had no effect on metastasis by SBC-3 or SBC-3 / ADM cells, combined use of MS-209 with VP-16 or ADM resulted in marked inhibition of metastasis formation by SBC-3 / ADM cells to multiple organs [2].
Dofequidar fumarate is a multidrug resistance (MDR)-reversing quinoline derivative that interacts directly with P-glycoprotein and inhibits the efflux of antitumor agents. The agent had been in phase III clinical development by Nihon Schering (now Bayer) for the treatment of advanced and recurrent breast cancer and non-small lung cancer (NSCLC) and at the National Cancer Institute in combination with docetaxel for the treatment of solid tumors. In 2000, Schering AG obtained dofequidar fumarate when Nihon Schering acquired Mitsui Pharmaceuticals, originator of the compound.
PAPER
Structure-activity relationship of newly synthesized quinoline derivatives for reversal of multidrug resistance in cancer
J Med Chem 1997, 40(13): 2047
J Med Chem 1997, 40(13): 2047
5-[3-{4-(2,2-Diphenylacetyl)piperazin-1-yl}-2-hydroxypropoxy]quinoline 1.5Fumarate (16, MS-209)
free form of 16 (7.37 g, 70%): mp 161−162 °C; 1H-NMR (CDCl3) δ 2.2−2.8 (m, 6 H), 3.5−3.6 (m, 2H), 3.7−3.9 (m, 2H), 4.1−4.3 (m, 3H), 5.20 (s, 1H), 6.86 (d, 1H,J = 7.3 Hz), 7.2−7.4 (m, 11H), 7.59 (t, 1H, J = 8.1 Hz), 7.71 (d, 1H, J = 8.1 Hz), 8.54 (d, 1H, J = 7.3 Hz), 8.91 (dd, 1H, J = 2, 4 Hz); IR (KBr) 2954, 1630, 1587, 1268, 1091, 802, 748, 703 cm-1.
16 1.5Fumarate(1.0 g, 60%): mp 210 °C dec; 1H-NMR (DMSO-d6) δ 2.2−2.6 (m, 6H), 3.4−3.6 (m, 4H), 4.0−4.2 (m, 3H), 5.53 (s, 1H), 6.63 (s, 3H), 7.03 (d, 1H, J = 8.1 Hz), 7.2−7.4 (m, 10H), 7.5−7.7 (m, 3H), 8.61 (d, 1H, J = 8.1 Hz), 8.89 (dd, 1H, J = 1.5, 4.4 Hz); IR (KBr) 3424, 1644, 1592, 1277, 1180, 1110, 799 cm-1.
Patent
WO 2004099151
A method for producing the purest rac-1 – {4- [2-hydroxy-3- (5-quinolyloxy) propyI] -piperazin-1-yl} -2,2-diphenylethan-1-one fumarate and the purest rac-1 – {4- [2-hydroxy-3- (5-quinoly loxy) propylene l] piperazin-1-yl} -2,2-diphenylethan-1 -one fumarate
The invention relates to a method for producing the purest rac-1 – {4- [2-hydroxy-3- (5-quinolyloxy) propyl] -piperazin-1-yl} -2,2-diphenylethan-1-one fumarate as well as rac -1- {4- [2- hydroxy-3- (5-quinolyloxy) propyl] piperazin-1-yl} -2,2-diphenylethan-1-one fumarate with a purity of at least 99.55%
The multidrug resistance modulator rac-1 – {4- [2-hydroxy-3- (5-quinolyloxy) propyl] – piperazin-1-yl} -2,2-diphenylethan-1 -one fumarate, its preparation and use as carcinostatic drug is described as well as other derivatives of this compound in EP 575,890.
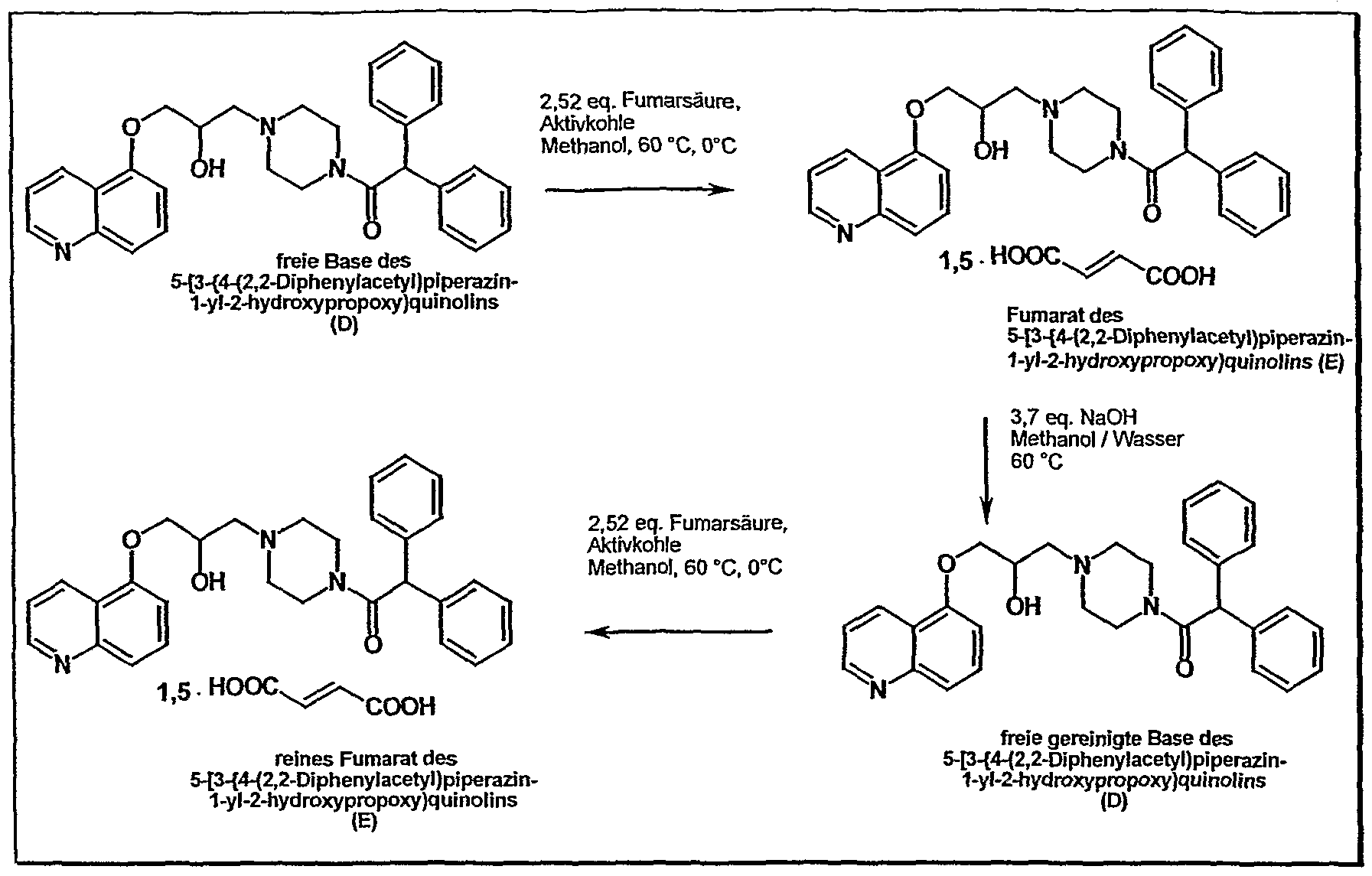
According to the process described in EP 575 890 A process for the preparation of pure rac-1 – {4- [2-hydroxy-3- (5-quinolyloxy) propyl] -piperazin-1-yl} -2,2-dϊphenylethan-1-one fumarate is first by coupling the two modules epoxiline (B) (5- (2,3-epoxypropoxy) – quinoline) and Diphenpiperazid (C) (N- (2,2-Diphenylacetyl) piperazine), the free base 5- [3- {4- (2,2-diphenylacetyl) piperazin-1-yl} -2-hydroxypropoxy] quinoline isolated as a crude product. This implementation includes two sub-stages. First, the Epoxylat with hydroxyquinoline (A) is reacted. In the second step the epoxiline (B) (5- (2,3-epoxypropoxy) -quinolin) by Diphenpiperazid (C) (N- (2,2-Diphenylacetyl) piperazine) is opened, it gives the secondary alcohol (D). This reaction takes place in ethanol, water catalyzes the conversion. The workup / isolation is then carried out by precipitation from acetone / water and drying under vacuum at 60 ° C.
The overall reaction results from the following scheme:
On the isolation of the free base, the many impurities (purity of the crude product is typically about 80%), joins in the next step a very expensive cleaning procedures. After charcoal treatment of the free base and the formation of the fumarate in methanol, the free base is again prepared by treatment with dilute sodium hydroxide solution for purification. Subsequently, as the last step, repeated fumarate formation. The two fumarate formations are procedurally identical and differ only in the batch size (T. Suzuki et al., J. Med. Chem. (1997) 40, 2047) (JP 2000281653). Starting from the crude free base, the typical yield for this laboratory cleaning sequence 45% of theory.
A disadvantage of this method is not only the low yield (about 50% loss in the final stage), but also the complex technical implementation, which binds many operational capacities and thus caused increased costs. A particular disadvantage is the extremely poor filterability of the free base, the filter must be dried partially over several weeks.
Despite the high procedural expenses according to this known method, the extremely high purity requirements of rac-1 – {4- [2-hydroxy-3- (5- quinolyloxy) propyl] piperazine-1-yl} -2,2-diphenylethane-1 -one fumarate not always be achieved completely satisfactory.
. Furthermore provides the method described in EP 575 890 any reasonable results during scale-up an overview of the individual reactions are the following scheme:
It has now been found that these known disadvantages can be overcome with the process of this invention. In the process of this invention also the epoxiline (B) and Diphenpiperazid (C) is first coupled by opening of the epoxide. But is not the free base (D) but after the addition of solid fumaric acid directly the fumarate salt (E) is then isolated as a crude product.
The present application thus provides a process for the preparation of pure rac-1 – {4- [2-hydroxy-3- (5-quinolyloxy) propyl] -piperazin-1-yl} -2,2-diphenylethan-1 -one fumarate , which is characterized in that firstly
a) a Epoxytosylat of structure I
OTs
(0 with
b) 5-hydroxyquinoline (II)
(II) and cesium carbonate in a suitable solvent and at a suitable temperature to 5- (2,3-epoxypropoxy) -quinolin of formula III
allowed to react, and then the 5- (2,3-epoxypropoxy) -quinolin of formula III
c) with N- (2,2-Diphenylacefyl) piperazine of the formula IV
in a suitable solvent and at a suitable temperature followed by the addition of solid fumaric acid to the crude rac-1 – {4- [2-hydroxy-3- (5-quinolyloxy) propyl] – piperazin-1-yl} -2,2-diphenylethane 1-one fumarate of the formula V
And subsequently reacting (V)
d) the thus formed crude rac-1 – fumarate {4- [2-hydroxy-3- (5-quinolyloxy) propyl] -piperazin-1-yl} -2,2-diphenylethan-1 -one (V) is isolated and is dissolved in a solvent mixture of methanol and methylene chloride, is treated with activated carbon and subsequently filtered through a pressure filter having silica gel as column material, and the thus obtained pure rac-1 – {4- [2-hydroxy-3- (5-quinolyloxy) propyl] -piperazin-1-yl} -2,2-diphenylethan-1-one fumarate (V) is crystallized from a suitable alcohol.
Preparation Example
Preparation of rac-1 – 4- [2-Hy droxy-3- (5-quinolyloxy) propylene l] -piperazin-1 -yl> -2,2-diphenylethan-1-one fumarate
A) Under nitrogen, 44.2 g of 5-hydroxy-quinoline and 151.9 g of cesium carbonate with 560 ml acetone will give at room temperature together and stirred for 30 minutes at 60 ° C bath temperature. At 50 ° C internal temperature 73.0 g of 5- (2,3-epoxypropoxy) -quinolin dissolved in
153.3 g of dichloromethane, admit. The mixture is stirred at 50 ° C for two hours.The mixture is filtered at 50 ° C. The filter residue (inorganic salts) is washed with 560 ml of 50 ° C warmed acetone. 85.4 g are then N- (2,2-diphenyl-acetyl) piperazine admit and concentrated at a bath temperature of 40 ° C under vacuum to 374 g final weight. It will then add 374 g of demineralized water and 2
Stirred at 40 ° C hours. Then 255 g of acetone and 201 g of demineralized water will admit. The mixture is cooled to room temperature and 89.1 g of fumaric acid are in solid form to Gege-ben. It is stirred for 60 minutes at 60 ° C bath temperature and then stirred at 0 ° C for 2 hours. The solid is suction filtered and washed with 150 ml of ice-cold methanol. The filter residue is dried at 60 ° C under vacuum.
Yield: 65 – 85% of theory
B) 56.0 g of the thus prepared rac-1 – {4- [2-hydroxy-3- (5-quinolyIoxy) propyl] -piperazin-1-yl} – 2,2-diphenylethan-1-one fumarate were nitrogen and treated at room temperature with 5.6 g of activated carbon, Norit SX plus, 672 ml of methanol and 1008 ml of dichloromethane. The resulting suspension is stirred at a bath temperature of 75 ° C to warm to reflux temperature and refluxed for 30 min. At an internal temperature of 40 ° C is rac-1 – {4- [2-hydroxy-3- (5-quinolyloxy) propyl] -piperazin-1-yl} -2,2-diphenylethan-1-one fumarate in solution. The mixture is then filtered hot through 300% silica gel and the silica gel with 560 ml of a mixture of 168 ml of methanol and 392 ml of dichloromethane at room temperature RT. The solution is concentrated at a bath temperature of 40 ° C and an initial vacuum of 400 mbar to a final volume of 517 ml. The ultimate vacuum of 350 mbar. The distilled volume is about the difference in volume (about 1, 7 I). There are 404 ml of methanol was added so that a final volume of 921 ml is achieved. The solution is cooled to 0 ° C, whereupon the product precipitates. The resulting suspension is stirred for 2 hours at 0 ° C and then filtered through a paper filter. The filter residue is washed with 56.0 ml of ice-cold methanol. The filter residue is dried at 60 ° C and under vacuum at 100 mbar for 10 hours.
Yield (. Uncorr): 47.29 g (84.45% FS)
Purity: 99.65% (HPLC, 100% method)
References on Dofequidar fumarate
http://jco.ascopubs.org/content/25/4/411.full.pdf
SEE.............http://newdrugapprovals.org/2016/01/08/16608/
SEE.............http://newdrugapprovals.org/2016/01/08/16608/
///////////MS-209, Dofequidar fumarate, PHASE 3






No comments:
Post a Comment