 .
.Picture credit....Bethany Halford
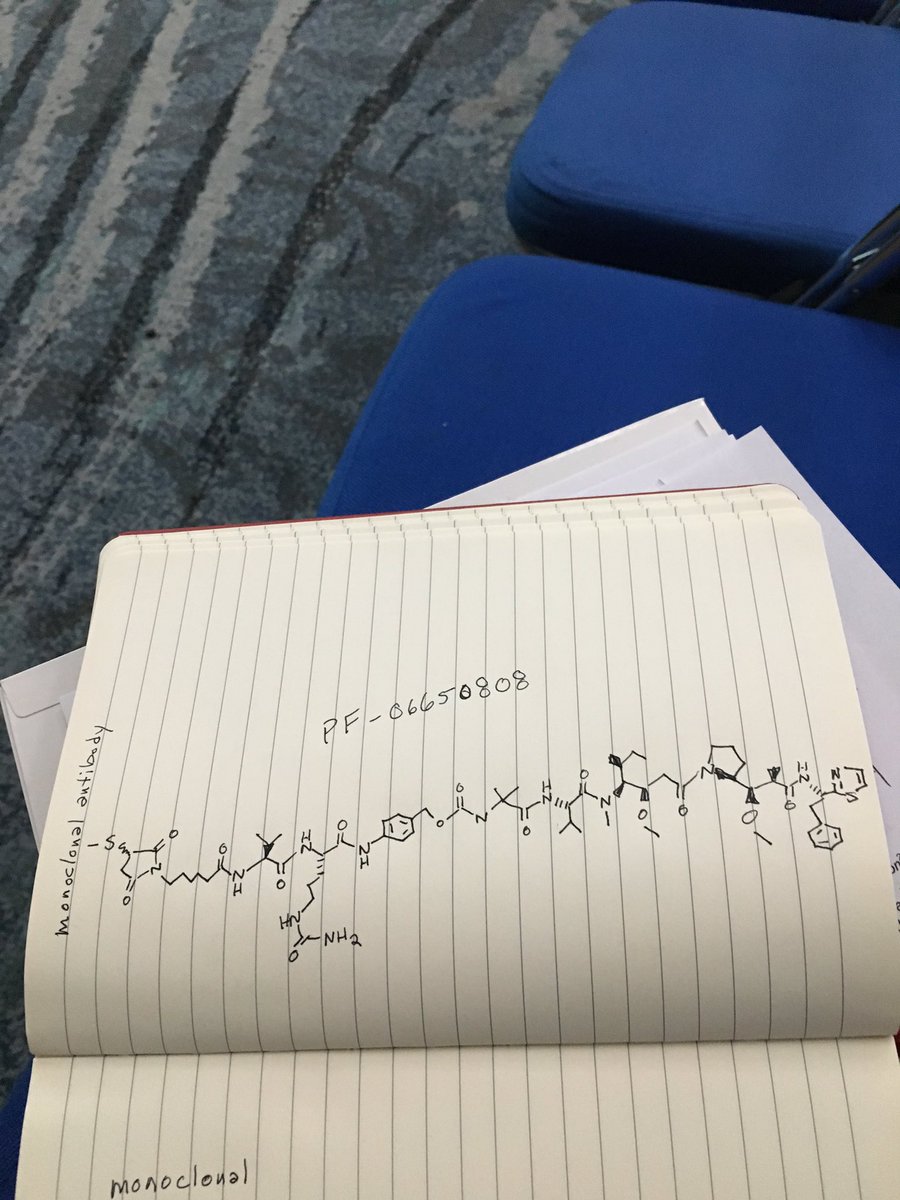
PF 06650808
| Phase 1 |
https://clinicaltrials.gov/ct2/show/NCT02129205
http://www.pfizer.com/sites/default/files/product-pipeline/8_7_2014_Pipeline_Update.pdf
ALL DATA COMING.........
Notch-3 receptor antagonists
Neoplasms
Breast
| Pfizer |
PF-06650808, is currently being examined in a Ph1 clinical trial (Protocol B7501001).
Notch3
Researchers are also exploring the use of Notch3 targeting. “The Notch pathway plays an important role in the growth of several solid tumours, including breast and ovarian cancer and melanoma,” explained Joerger. “In particular, Notch3 alterations such as gene amplification and upregulation are associated with poor patient survival. Research using Notch3 targeting as an innovative approach to treat solid malignancies included 27 patients unselected for Notch3 who received increasing doses of the anti-Notch3 antibody-drug conjugate PF-06650808. Responses were seen in two breast cancer patients (LBA 30). While preliminary, targeting Notch3 may become a new treatment approach in patients with selected solid tumours.”
The anti-Notch3 antibody-drug conjugate PF-06650808 is being developed by Pfizer.
- 31 Jul 2014 Phase-I clinical trials in Solid tumours (Late-stage disease) in USA (Parenteral)
- 30 Apr 2014 Preclinical trials in Solid tumours in USA (Parenteral)
- 30 Apr 2014 Pfizer plans a phase I trial for Solid tumours (late-stage disease, second-line therapy or greater) in USA (NCT02129205)


/////////PF 06650808, PF-06650808, PF-6650808, monoclonal antibody, pfizer, phase 1, Solid tumours , Notch-3 receptor antagonists
C1(C(N(C(C1)=O)CCCCCC(=O)NC([C@H](C)C)C(=O)NC(C(=O)Nc2ccc(cc2)COC(=O)NC(C)(C)C(=O)N[C@@H](C(C)C)C(=O)[N@](C)C(C(CC)C)[C@@H](OC)CC(=O)N3CCC[C@H]3C(OO)C(C)C(=O)N[C@H](c4nccs4)CC)CCCNC(=O)N)=O)SC
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
 amcrasto@gmail.com
amcrasto@gmail.com
P.STHE VIEWS EXPRESSED ARE MY PERSONAL AND IN NO-WAY SUGGEST THE VIEWS OF THE PROFESSIONAL BODY OR THE COMPANY THAT I REPRESENT, amcrasto@gmail.com, +91 9323115463 India.
I , Dr A.M.Crasto is writing this blog to share the knowledge/views, after reading Scientific Journals/Articles/News Articles/Wikipedia. My views/comments are based on the results /conclusions by the authors(researchers). I do mention either the link or reference of the article(s) in my blog and hope those interested can read for details. I am briefly summarising the remarks or conclusions of the authors (researchers). If one believe that their intellectual property right /copyright is infringed by any content on this blog, please contact or leave message at below email address amcrasto@gmail.com. It will be removed ASAP


No comments:
Post a Comment