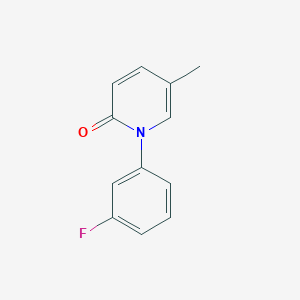
Vibegron, MK-4618, KRP114V
UNII-M5TSE03W5U; M5TSE03W5U; D10433
Molecular Formula: C26H28N4O3 Molecular Weight: 444.52552
phase 2 for the treatment of overactive bladder
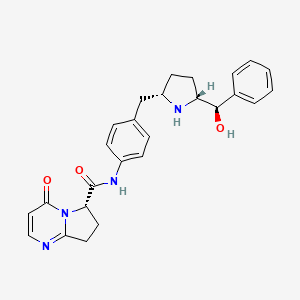
(6S)-N-[4-([(2S,5R)-5-[(R)-Hydroxy(phenyl)methyl]pyrrolidin-2-yl]methyl)phenyl]-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxamide
(6S)-N-[4-[[(2S,5R)-5-[(R)-hydroxy(phenyl)methyl]pyrrolidin-2-yl]methyl]phenyl]-4-oxo-7,8-dihydro-6H-pyrrolo[1,2-a]pyrimidine-6-carboxamide
Target-based Actions Beta 3 adrenoceptor agonist
Indications Overactive bladder; Urinary incontinence
Kyorin Pharmaceutical, under license from Merck, is developing
vibegron (phase II, September 2014) for the treating of overactive
bladder. In July 2014, Merck has granted to Kyorin an exclusive license
to develop, manufacture and commercialize vibegron in Japan.
MK-4618 is being developed in phase II clinical trials at Merck &
Co. for the treatment of overactive bladder. The company had been
developing the compound for the treatment of endocrine disorders and
hypertension; however, recent progress reports are not available at
present.
In 2014, Merck licensed the product to Kyorin for development and commercialization in Japan.
The function of the lower urinary tract is to store and periodically
release urine. This requires the orchestration of storage and
micturition reflexes which involve a variety of afferent and efferent
neural pathways, leading to modulation of central and peripheral
neuroeffector mechanisms, and resultant coordinated regulation of
sympathetic and parasympathetic components of the autonomic nervous
system as well as somatic motor pathways. These proximally regulate the
contractile state of bladder (detrusor) and urethral smooth muscle, and
urethral sphincter striated muscle.
β Adrenergic receptors (βAR) are present in detrusor smooth muscle of
various species, including human, rat, guinea pig, rabbit, ferret, dog,
cat, pig and non-human primate. However, pharmacological studies
indicate there are marked species differences in the receptor subtypes
mediating relaxation of the isolated detrusor; β1AR predominate in cats
and guinea pig, β2AR predominate in rabbit, and β3AR contribute or
predominate in dog, rat, ferret, pig, cynomolgus and human detrusor.
Expression of βAR subtypes in the human and rat detrusor has been
examined by a variety of techniques, and the presence of β3AR was
confirmed using in situ hybridization and/or reverse
transcription-polymerase chain reaction (RT-PCR). Real time quantitative
PCR analyses of β1AR, β2AR and β3AR mRNAs in bladder tissue from
patients undergoing radical cystectomy revealed a preponderance of β3AR
mRNA (97%, cf 1.5% for β1AR mRNA and 1.4% for β2AR mRNA). Moreover, β3AR
mRNA expression was equivalent in control and obstructed human
bladders. These data suggest that bladder outlet obstruction does not
result in downregulation of β3AR, or in alteration of β3AR-mediated
detrusor relaxation. β3AR responsiveness also has been compared in
bladder strips obtained during cystectomy or enterocystoplasty from
patients judged to have normal bladder function, and from patients with
detrusor hyporeflexia or hyperreflexia. No differences in the extent or
potency of β3AR agonist mediated relaxation were observed, consistent
with the concept that the β3AR activation is an effective way of
relaxing the detrusor in normal and pathogenic states.
Functional evidence in support of an important role for the β3AR in
urine storage emanates from studies in vivo. Following intravenous
administration to rats, the rodent selective β3AR agonist CL316243
reduces bladder pressure and in cystomeric studies increases bladder
capacity leading to prolongation of micturition interval without
increasing residual urine volume.
Overactive bladder is characterized by the symptoms of urinary
urgency, with or without urgency urinary incontinence, usually
associated with frequency and nocturia. The prevalence of OAB in the
United States and Europe has been estimated at 16 to 17% in both women
and men over the age of 18 years. Overactive bladder is most often
classified as idiopathic, but can also be secondary to neurological
condition, bladder outlet obstruction, and other causes. From a
pathophysiologic perspective, the overactive bladder symptom complex,
especially when associated with urge incontinence, is suggestive of
detrusor overactivity. Urgency with or without incontinence has been
shown to negatively impact both social and medical well-being, and
represents a significant burden in terms of annual direct and indirect
healthcare expenditures. Importantly, current medical therapy for
urgency (with or without incontinence) is suboptimal, as many patients
either do not demonstrate an adequate response to current treatments,
and/or are unable to tolerate current treatments (for example, dry mouth
associated with anticholinergic therapy). Therefore, there is need for
new, well-tolerated therapies that effectively treat urinary frequency,
urgency and incontinence, either as monotherapy or in combination with
available therapies. Agents that relax bladder smooth muscle, such as
β3AR agonists, are expected to be effective for treating such urinary
disorders.
PATENT
http://www.google.com/patents/WO2013062881A1?cl=en

EXAMPLE 3
To a three neck flask equipped with a N
2 inlet, a thermo
couple probe was charged pyrrolidine i-11 (10.0 g), sodium salt i-12
(7.87 g), followed by IPA (40 mL) and water (24 mL). 5 N HC1 (14.9 mL)
was then slowly added over a period of 20 min to adjust pH = 3.3- 3.5,
maintaining the batch temperature below 35 °C. Solid EDC hydrochloride
(7.47 g) was charged in portions over 30 min. The reaction mixture was
aged at RT for additional 0.5 – 1 h, aqueous ammonia (14%) was added
dropwise to pH ~8.6. The batch was seeded and aged for additional 1 h to
form a slurry bed. The rest aqueous ammonia (14%, 53.2 ml total) was
added dropwise over 6 h. The resulting thick slurry was aged 2-3 h
before filtration. The wet-cake was displacement washed with 30% IPA (30
mL), followed by 15% IPA (2 x 20mL) and water (2 X 20mL). The cake was
suction dried under N
2 overnight to afford 14.3 g of compound of Formula (I)-
1H NMR (DMSO) δ 10.40 (s, NH), 7.92 (d, J = 6.8, 1H), 7.50 (m, 2H),
7.32 (m, 2H), 7.29 (m, 2H), 7.21 (m, 1H), 7.16 (m, 2H), 6.24 (d, J =
6.8, 1H), 5.13 (dd, J = 9.6, 3.1, 1H), 5.08 (br s, OH), 4.22 (d, J =
7.2, 1H), 3.19 (p, J = 7.0, 1H), 3.16-3.01 (m, 3H), 2.65 (m, 1H),
2.59-2.49 (m, 2H), 2.45 (br s, NH), 2.16 (ddt, J = 13.0, 9.6, 3.1, 1H),
1.58 (m, 1H), 1.39 (m, 1H), 1.31-1.24 (m, 2H).
13C NMR (DMSO) δ 167.52, 165.85, 159.83, 154.56, 144.19,
136.48, 135.66, 129.16, 127.71, 126.78, 126.62, 119.07, 112.00, 76.71,
64.34, 61.05, 59.60, 42.22, 31.26, 30.12, 27.09, 23.82.
HPLC method – For monitoring conversion
Column: XBridge C18 cm 15 cm x 4.6 mm, 3.5 μιη particle size;
Column Temp. : 35 °C; Flow rate: 1.5 mL/min; Detection: 220 nm;
Mobile phase: A. 5 mM Na
2B40
7.10 H20 B: Acetonitrile
Gradient:
HPLC method – For level of amide epimer detection
Column: Chiralpak AD-H 5 μηι, 250 mm x 4.6 mm.
Column Temp: 35 °C; Flow rate: 1.0 mL/min; Detection: 250 nm;
Mobile phase: Isocratic 30% Ethanol in hexanes + 0.1% isobutylamine
PATENT
WO 2009124167
http://www.google.com/patents/WO2009124167A1?cl=en
EXAMPLE 103
(6y)-N-r4-({(
‘25′. 5R)-5-r(
‘R)-hvdroxy(
‘phenvnmethyl1pyrrolidin-2-yl}methvnphenyl1-4-oxo- 4,6J,8-tetrahydropyiτolori,2-α1pyrimidine-6-carboxamide
ter?-butyl(2R. 55
f)-2-rCR)-hvdroxy(
‘phenvnmethyl1-5-r4-(
‘{r(
‘65
f)-4-oxo-4.6.7.8-
tetrahydropyrrolof 1.2-alpyrimidin-6- yl]carbonyl} amino)benzyl]pyrrolidine- 1 – carboxylate
To a solution of i-13a (21.4 g, 55.9 mmol) in N,N-dimethylformamide (100 ml) at O
0C
was added
[(65)-4-oxo-4,6,7,8-tetrahydropyrrolo[l,2-α]pyrimidine-6-carboxylic acid
(11.1 g, 61.5 mmol), followed by 1 -hydroxybenzotriazole (i-44, 7.55 g,
55.9 mmol), N-(3- dimethylaminopropyl)-N
l-ethylcarbodiimide
hydrochloride (16.1 g, 84.0 mmol) and N,N- diisopropylethylamine (29.2
ml, 168 mmol). The reaction mixture was stirred from O
0C to
ambient temperature for 2 h. Water (600 ml) was added and it was
extracted with dichloromethane (600 ml x 2). The combined organic layers
were dried over Na
2SO
4. After removal of the volatiles, the residue was purified by using a Biotage Horizon®
system (0-5% then 5% methanol with 10% ammonia/dichloromethane mixture)
to afford the title compound which contained 8% of the minor
diastereomer. It was further purified by supercritical fluid
chromatography (chiral AS column, 40% methanol) to afford the title
compound as a pale yellow solid (22.0 g, 72%).
1H NMR (CDCl
3):
δ 9.61 (s, IH), 7.93 (d, J = 6.6 Hz, IH), 7.49 (d, J = 8.4 Hz, 2H),
7.35-7.28 (m, 5H), 7.13 (d, J = 8.5 Hz, 2H), 6.40 (d, J = 6.7 Hz, IH),
5.36 (d, J = 8.6 Hz, IH), 4.38 (m, IH), 4.12-4.04 (m, 2H), 3.46 (m,lH),
3.15-3.06 (m, 2H), 2.91 (dd, J = 13.1, 9.0 Hz, IH), 2.55 (m, IH), 2.38
(m, IH), 1.71-1.49 (m, 13H). LC-MS 567.4 (M+23).
(6S)-N-\4-( U2S. 5R)-5-r(R)-hvdroxy(phenyl)methyl1pyrrolidin-2-
yl}methyl)phenyl1-4-oxo-4,6J,8-tetrahvdropyrrolori,2-α1pyrimidine-6- carboxamide
To a solution of the intermediate from Step A (2.50 g, 4.59 mmol) in
dichloromethane (40 ml) was added trifluoroacetic acid (15 ml). The
reaction mixture was stirred at ambient temperature for 1.5 h. After
removal of the volatiles, saturated NaHCCh was added to make the PH
value to 8-9. The mixture was then extracted with dichloromethane. The
combined organic layers were dried over Na
2SO
4. After concentration, crystallization from methanol/acetonitrile afforded the title compound as a white solid (1.23g, 60%).
1H NMR (DMSO-Cl
6):
δ 10.40 (s, IH), 7.91 (d, J = 6.7 Hz, IH), 7.49 (d, J = 8.3 Hz, 2H),
7.32-7.26 (m, 4H), 7.21 (m, IH), 7.15 (d, J = 8.4 Hz, 2H), 6.23 (d, J =
6.7 Hz, IH), 5.11 (dd, J = 9.6, 2.9 Hz, IH), 5.10 (br, IH), 4.21 (d, J =
7.1 Hz, IH), 3.20-3.00 (m, 4H), 2.66-2.51 (m, 3H), 2.16 (m, IH), 1.57
(m, IH), 1.38 (m, IH), 1.29-1.23 (m, 2H). LC-MS 445.3 (M+l).
Using the Biological Assays described above, the human β3 functional
activity of Example 103 was determined to be between 11 to 100 nM.
PATENT
CHECK STRUCTURE…………….CAUTION
http://www.google.com/patents/US8247415


CAUTION…………….
Example
103(6S)-N-[4-({(2S,5R)-5-[(R)-hydroxy(phenyl)methyl]pyrrolidin-2-yl}methyl)phenyl]-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]pyrimidine-6-carboxamide
Step A:
tert-butyl(2R,5S)-2-[(R)-hydroxy(phenyl)methyl]-5-[4-({[(6S)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]pyrimidin-6-yl]carbonyl}amino)benzyl]pyrrolidine-1-carboxylate
To a solution of i-13a (21.4 g, 55.9 mmol) in N,N-dimethylformamide
(100 ml) at 0° C. was added
[(6S)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]pyrimidine-6-carboxylic acid
(11.1 g, 61.5 mmol), followed by 1-hydroxybenzotriazole (i-44, 7.55 g,
55.9 mmol), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
(16.1 g, 84.0 mmol) and N,N-diisopropylethylamine (29.2 ml, 168 mmol).
The reaction mixture was stirred from 0° C. to ambient temperature for 2
h. Water (600 ml) was added and it was extracted with dichloromethane
(600 ml×2). The combined organic layers were dried over Na
2SO
4. After removal of the volatiles, the residue was purified by using a Biotage Horizon®
system (0-5% then 5% methanol with 10% ammonia/dichloromethane mixture)
to afford the title compound which contained 8% of the minor
diastereomer. It was further purified by supercritical fluid
chromatography (chiral AS column, 40% methanol) to afford the title
compound as a pale yellow solid (22.0 g, 72%).
1H NMR (CDCl
3):
δ 9.61 (s, 1H), 7.93 (d, J=6.6 Hz, 1H), 7.49 (d, J=8.4 Hz, 2H),
7.35-7.28 (m, 5H), 7.13 (d, J=8.5 Hz, 2H), 6.40 (d, J=6.7 Hz, 1H), 5.36
(d, J=8.6 Hz, 1H), 4.38 (m, 1H), 4.12-4.04 (m, 2H), 3.46 (m, 1H),
3.15-3.06 (m, 2H), 2.91 (dd, J=13.1, 9.0 Hz, 1H), 2.55 (m, 1H), 2.38 (m,
1H), 1.71-1.49 (m, 13H). LC-MS 567.4 (M+23).
Step B:
(6S)-N-[4-({(2S,5R)-5-[(R)-hydroxy(phenyl)methyl]pyrrolidin-2-yl}methyl)phenyl]-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]pyrimidine-6-carboxamide
To a solution of the intermediate from Step A (2.50 g, 4.59 mmol) in
dichloromethane (40 ml) was added trifluoroacetic acid (15 ml). The
reaction mixture was stirred at ambient temperature for 1.5 h. After
removal of the volatiles, saturated NaHCO
3 was added to make
the PH value to 8-9. The mixture was then extracted with
dichloromethane. The combined organic layers were dried over Na
2SO
4. After concentration, crystallization from methanol/acetonitrile afforded the title compound as a white solid (1.23 g, 60%).
1H NMR (DMSO-d
6):
δ 10.40 (s, 1H), 7.91 (d, J=6.7 Hz, 1H), 7.49 (d, J=8.3 Hz, 2H),
7.32-7.26 (m, 4H), 7.21 (m, 1H), 7.15 (d, J=8.4 Hz, 2H), 6.23 (d, J=6.7
Hz, 1H), 5.11 (dd, J=9.6, 2.9 Hz, 1H), 5.10 (br, 1H), 4.21 (d, J=7.1 Hz,
1H), 3.20-3.00 (m, 4H), 2.66-2.51 (m, 3H), 2.16 (m, 1H), 1.57 (m, 1H),
1.38 (m, 1H), 1.29-1.23 (m, 2H). LC-MS 445.3 (M+1).
Using the Biological Assays described above, the human β3 functional
activity of Example 103 was determined to be between 11 to 100 nM.
PATENT
WO2014150639
http://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014150639&recNum=4&docAn=US2014023858&queryString=EN_ALL:nmr%20AND%20PA:merck&maxRec=11148
Step 6. Preparation of Compound 1-7 from Compound 1-6 and Compound A-2

To a three neck flask equipped with a N
2 inlet, a thermo
couple probe was charged pyrrolidine hemihydrate 1-6 (10.3 g), sodium
salt A-2 (7.87 g), followed by IPA (40 mL) and water (24 mL). 5 N HC1
(14.9 mL) was then slowly added over a period of 20 minutes to adjust pH
= 3.3-3.5, maintaining the batch temperature below 35°C. Solid EDC
hydrochloride (7.47 g) was charged in portions over 30 minutes. The
reaction mixture was aged at RT for additional 0.5 – 1 hour, aqueous
ammonia (14%) was added dropwise to pH -8.6. The batch was seeded and
aged for additional 1 hour to form a slurry bed. The rest aqueous
ammonia (14%, 53.2 ml total) was added dropwise over 6 hours. The
resulting thick slurry was aged 2-3 hours before filtration. The
wet-cake was displacement washed with 30% IPA (30 mL), followed by 15%
IPA (2 x 20mL) and water (2 X 20mL). The cake was suction dried under N
2 overnight to afford 14.3 g of compound 1-7.
1H NMR (DMSO) δ 10.40 (s, NH), 7.92 (d, J = 6.8, 1H), 7.50 (m, 2H),
7.32 (m, 2H), 7.29 (m, 2H), 7.21 (m, 1H), 7.16 (m, 2H), 6.24 (d, J =
6.8, 1H), 5.13 (dd, J = 9.6, 3.1, 1H), 5.08 (br s, OH), 4.22 (d, J =
7.2, 1H), 3.19 (p, J = 7.0, 1H), 3.16-3.01 (m, 3H), 2.65 (m, 1H),
2.59-2.49 (m, 2H), 2.45 (br s, NH), 2.16 (ddt, J = 13.0, 9.6, 3.1, 1H),
1.58 (m, 1H), 1.39 (m, 1H), 1.31-1.24 (m, 2H).
13C NMR (DMSO) δ 167.52, 165.85, 159.83, 154.56, 144.19,
136.48, 135.66, 129.16, 127.71, 126.78, 126.62, 119.07, 112.00, 76.71,
64.34, 61.05, 59.60, 42.22, 31.26, 30.12, 27.09, 23.82.
The crystalline freebase anhydrous form I of Compound 1-7 can be characterized by XRPD by

PATENT
WO-2014150633
Merck Sharp & Dohme Corp
Process for preparing stable immobilized ketoreductase comprises bonding
of recombinant ketoreductase to the resin in a solvent. Useful for
synthesis of vibegron intermediates. For a concurrent filling see
WO2014150639, claiming the method for immobilization of ketoreductase.
Picks up from WO2013062881, claiming the non enzymatic synthesis of
vibegron and intermediates.
PAPER
Discovery of Vibegron: A Potent and Selective β3 Adrenergic Receptor Agonist for the Treatment of Overactive Bladder
Merck Research Laboratories, 2015 Galloping Hill Road, PO Box 539, Kenilworth, New Jersey 07033, United States
J. Med. Chem., Article ASAP
DOI: 10.1021/acs.jmedchem.5b01372
Publication Date (Web): December 27, 2015
Copyright © 2015 American Chemical Society
http://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.5b01372
http://pubs.acs.org/doi/suppl/10.1021/acs.jmedchem.5b01372/suppl_file/jm5b01372_si_001.pdf
The discovery of vibegron, a potent and selective human β
3-AR agonist for the treatment of overactive bladder (OAB), is described. An early-generation clinical β
3-AR agonist MK-0634 (
3)
exhibited efficacy in humans for the treatment of OAB, but development
was discontinued due to unacceptable structure-based toxicity in
preclinical species. Optimization of a series of second-generation
pyrrolidine-derived β
3-AR agonists included reducing the risk
for phospholipidosis, the risk of formation of disproportionate human
metabolites, and the risk of formation of high levels of circulating
metabolites in preclinical species. These efforts resulted in the
discovery of vibegron, which possesses improved druglike properties and
an overall superior preclinical profile compared to MK-0634.
Structure–activity relationships leading to the discovery of vibegron
and a summary of its preclinical profile are described.
SYNTHESIS
A study of the efficacy and safety of MK-4618 in patients with overactive bladder (OAB) (MK-4618-008 EXT1) (NCT01314872)
ClinicalTrials.gov Web Site 2011, April 28
| WO2011043942A1 * |
Sep 27, 2010 |
Apr 14, 2011 |
Merck Sharp & Dohme Corp. |
Combination therapy using a beta 3 adrenergic receptor agonist and an antimuscarinic agent |
| US20090253705 * |
Apr 2, 2009 |
Oct 8, 2009 |
Richard Berger |
Hydroxymethyl pyrrolidines as beta 3 adrenergic receptor agonists |
| US20110028481 * |
Apr 2, 2009 |
Feb 3, 2011 |
Richard Berger |
Hydroxymethyl pyrrolidines as beta 3 adrenergic receptor agonists |
| Citing Patent |
Filing date |
Publication date |
Applicant |
Title |
| US8642661 |
Aug 2, 2011 |
Feb 4, 2014 |
Altherx, Inc. |
Pharmaceutical combinations of beta-3 adrenergic receptor agonists and muscarinic receptor antagonists |
| US8653260 |
Jun 20, 2012 |
Feb 18, 2014 |
Merck Sharp & Dohme Corp. |
Hydroxymethyl pyrrolidines as beta 3 adrenergic receptor agonists |
| US20120202819 * |
Sep 27, 2010 |
Aug 9, 2012 |
Merck Sharp & Dohme Corporation |
Combination therapy using a beta 3 adrenergic receptor agonists and an antimuscarinic agent |
|
|
8-22-2012
|
Hydroxymethyl pyrrolidines as [beta]3 adrenergic receptor agonists
|
////////////C1CC(NC1CC2=CC=C(C=C2)NC(=O)C3CCC4=NC=CC(=O)N34)C(C5=CC=CC=C5)O