ACH-3102 , Odalasvir
Odalasvir
ACH-0143102; ACH-3102
CAS : 1415119-52-6
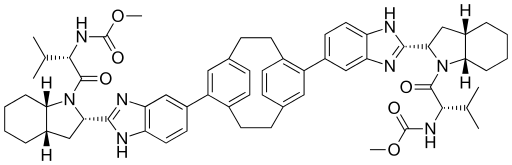
Dimethyl N, N ‘- (tricyclo [8.2.2.24,7] hexadeca-1 (12), 4,6, 10,13,15-hexaene-5,11-diylbis {1H-benzimidazole-5,2-diyl [(2S, 3aS, 7aS) -octahydro-1H-indole-2,1-diyl] [(1S) -1 – (1-methylethyl) -2-oxoethylene]}) biscarbamate
Carbamic acid, N,N’-(tricyclo(8.2.2.24,7)hexadeca-4,6,10,12,13,15-hexaene-5,11-diylbis(1H-benzimidazole-6,2-diyl((2S,3aS,7aS)-octahydro-1H-indole-2,1-diyl)((1S)-1-(1-methylethyl)-2-oxo-2,1-ethanediyl)))bis-, C,C’-dimethyl ester
Dimethyl N,N’-(1,4(1,4)-dibenzenacyclohexaphane-12,42-diylbis(1hbenzimidazole-5,2-diyl((2S,3aS,7aS)-octahydro-1H-indole-2,1-diyl)((2S)-3-methyl-1-oxobutan-1,2-diyl)))biscarbamate
Mechanism of Action: HCV NS5A Protein inhibitor
Indication: Hepatitis C
Developer: Achillion Pharmaceuticals, Inc.
-
C60-H72-N8-O6
- 1001.2788
Achillion Pharmaceuticals Inc’s Odalasvir (ACH-3102) is an investigational new drug in development for the treatment hepatitis C. Achillion’s ongoing study tests its NS5A inhibitor, ACH-3102, with Sovaldi in previously untreated genotype 1 hepatitis C patients over six and eight weeks of therapy. The main goal is to achieve a cure, or sustained virological response, 12 weeks after the completion of therapy.
Odalasvir is a hepatitis C virus (HCV NS5A) inhibitor in phase II clinical studies at Achillion for the treatment of hepatitis C.
In 2012, fast track designation was assigned to the compound in the U.S. for the treatment of chronic hepatitis C.
WILL BE UPDATED………….
WO 2012166716
http://www.google.com/patents/US20120302538
General Considerations
All nonaqueous reactions were performed under an
atmosphere of dry argon gas using oven-dried glassware and anhydrous
solvents. The progress of reactions and the purity of target compounds
were determined using one of the following two HPLC methods: (1) Waters
AQUITY HPLC BEH C18 1.7 μm 2.1×50 mm column with an isocratic elution of
0.24 min at 90:10 water:acetonitrile containing 0.05% formic acid
followed by a 4.26-min linear gradient elution from 90:10 to 10:90 at a
flow rate of 1.0 mL/min with UV (PDA), ELS, and MS (SQ in APCI mode)
detection (method 1); and (2) Waters AQUITY HPLC BEH C18 1.7 μm 2.1×50
mm column with an isocratic elution of 0.31 min at 95:5
water:acetonitrile containing 0.05% formic acid followed by a 17.47-min
linear gradient elution from 95:5 to 5:95 at a flow rate of 0.4 mL/min
with UV (PDA), ELS, and MS (SQ in APCI mode) detection (method 2).
Target compounds were purified via preparative
reverse-phase HPLC using a YMC Pack Pro C18 5 μm 150×20 mm column with
an isocratic elution of 0.35 min at 95:5 water:acetonitrile containing
0.1% trifluoroacetic acid followed by a 23.3-min linear gradient elution
from 95:5 to 5:95 at a flow rate of 18.9 mL/min with UV and mass-based
fraction collection.
Example 1
Synthesis of Compound 10
SEE ALSO
Compound 10 was prepared via bromination of [2.2]paracyclophane as outlined previously (Reich, H. J.; Cram, D. J. J. Am. Chem. Soc. 1969, 91, 3527-3533; Reich, H. J.; Cram, D. J. J. Am. Chem. Soc. 1969,
91, 3534-3543). Compounds 1, 2, 6, 8, and 10 can be obtained from
commercial sources. Compounds 3-7 and 9 were prepared using general
synthetic methods known in the art.
Example 2Synthesis of Compound 11
A deoxygenated (argon) mixture of 9 (284.2 mg), 10 (52.3 mg), K3PO4 (248.1 mg), and PdCl2dppf.CH2Cl2 (7.4
mg) in dioxane/water (5.5 mL/0.55 mL) was irradiated in a microwave for
2 h at 80° C. The resulting mixture was evaporated under reduced
pressure and the remaining solid was extracted with DCM. This crude
material was purified by PTLC (20 cm×20 cm×2000 μm glass plates; eluted
with 45:50:5 v/v/v DCM:EtOAc:MeOH, Rf 0.28) to give 75.3 mg
of 11. The purity of 11 was determined via analytical reverse-phase HPLC
using a 3.5-min gradient elution of increasing concentrations of ACN in
water (10-90%) containing 0.05% formic acid with a flow rate of 1.0
mL/min on a Waters AQUITY HPLC BEH C18 1.7 μm 2.1×50 mm column with UV
(PDA), ELS, and MS (SQ in APCI mode) detection. HPLC: tR 1.57 min (98% purity). MS m/z calculated for C56H64N8O6 ([M]+), 945. found, 946 ([M+1]+).
US 2012302538
http://www.google.com/patents/US20120302538
……………
see
US 20150023913
http://www.google.com/patents/US20150023913
…………..
see
WO 2015005901
https://patentscope.wipo.int/search/en/detail.jsf;jsessionid=7B94F69052D90AA41E2DAED2AE82A5C0.wapp1nA?docId=WO2015005901&recNum=76&maxRec=2577841&office=&prevFilter=&sortOption=&queryString=&tab=PCTDescription
 |
|
| Systematic (IUPAC) name | |
|---|---|
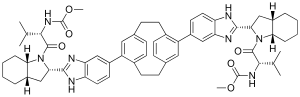
Dimethyl N,N′-(1,4(1,4)-Dibenzenacyclohexaphane-12,42-diylbis(1hbenzimidazole-5,2-diyl((2S,3aS,7aS)-octahydro-1H-indole-2,1-diyl)((2S)-3-methyl-1-oxobutan-1,2-diyl)))biscarbamate
|
|
| Clinical data | |
| Legal status |
|
| Identifiers | |
| CAS Registry Number | 1415119-52-6 |
| ATC code | None |
| Chemical data | |
| Formula | C60H72N8O6 |
| Molecular mass | 1001.28 g/mol |
सुकून उतना ही देना प्रभू, जितने से जिंदगी चल जाये। औकात बस इतनी देना, कि औरों का भला हो जाये।
Join me on google plus  Googleplus
Googleplus
 amcrasto@gmail.com
amcrasto@gmail.com
 LIONEL MY SON
LIONEL MY SON
He was only in first standard in school when I was hit by a deadly one in a million spine stroke called acute transverse mylitis, it made me 90% paralysed and bound to a wheel chair, Now I keep him as my source of inspiration and helping millions, thanks to millions of my readers who keep me going and help me to keep my son happy
सुकून उतना ही देना प्रभू, जितने से
जिंदगी चल जाये।
औकात बस इतनी देना,
कि औरों का भला हो जाये।
जिंदगी चल जाये।
औकात बस इतनी देना,
कि औरों का भला हो जाये।
No comments:
Post a Comment