
TOLVAPTAN
的合成
N-(4-{[(5R)-7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]carbonyl}-3-methylphenyl)-2-methylbenzamide| Formula | C26H25ClN2O3 |
|---|---|
| Mol. mass | 448.941 g/mol |
OPC-41061
Otsuka.....innovator
European Medicines Agency (EMA) Accepts Otsuka's Marketing Authorisation Application (MAA) for Tolvaptan, an Investigational Compound for Autosomal Dominant Polycystic Kidney Disease (ADPKD)
•Tolvaptan was discovered by Otsuka in Japan and, if approved by the EMA, would become the first pharmaceutical therapy in Europe for patients with ADPKD
•ADPKD is an inherited genetic disease that causes cyst growth in the kidneys, which gradually impairs their functioning. There is no current pharmaceutical treatment option
•Otsuka’s development of tolvaptan as a treatment for ADPKD illustrates the company’s commitment to address significant patient needs for diseases that traditionally have not been a priority for the pharmaceutical industry
TOKYO--(BUSINESS WIRE)--Otsuka Pharmaceutical Co., Ltd. announced today that the European Medicines Agency (EMA) has accepted the submission of a marketing authorisation application (MAA) for the potential approval of tolvaptan for the treatment of autosomal dominant polycystic kidney disease (ADPKD). Phase III clinical trial results that form the basis of the regulatory filing were published in the New England Journal of Medicine.
http://www.pharmalive.com/ema-accepts-otsukas-maa-for-tolvaptan
Tolvaptan is a selective vasopressin V2-receptor antagonist with an affinity for the V2-receptor that is 1.8 times that of native arginine vasopressin (AVP).
Tolvaptan is (±)-4'-[(7-chloro-2,3,4,5-tetrahydro-5-hydroxy-1H-1-benzazepin-1-yl) carbonyl]-otolu-m-toluidide. The empirical formula is C26H25ClN2O3. Molecular weight is 448.94. The chemical structure is:
 |
Tolvaptan (INN), also known as OPC-41061, is a selective, competitive vasopressin receptor 2 antagonist used to treat hyponatremia (low blood sodium levels) associated withcongestive heart failure, cirrhosis, and the syndrome of inappropriate antidiuretic hormone(SIADH). Tolvaptan was approved by the U.S. Food and Drug Administration (FDA) on May 19, 2009, and is sold by Otsuka Pharmaceutical Co. under the trade name Samsca and in India is manufactured & sold by MSN laboratories Ltd. under the trade name Tolvat & Tolsama.
Tolvaptan is also in fast-track clinical trials[2] for polycystic kidney disease. In a 2004 trial, tolvaptan, when administered with traditional diuretics, was noted to increase excretion of excess fluids and improve blood sodium levels in patients with heart failure without producing side effects such as hypotension (low blood pressure) or hypokalemia(decreased blood levels of potassium) and without having an adverse effect on kidney function.[3] In a recently published trial (TEMPO 3:4 ClinicalTrials.gov number, NCT00428948) the study met its primary and secondary end points. Tolvaptan, when given at an average dose of 95 mg per day over a 3-year period, slowed the usual increase in kidney volume by 50% compared to placebo (2.80% per year versus 5.51% per year, respectively, p<0.001) and reduced the decline in kidney function when compared with that of placebo-treated patients by approximately 30% (reciprocal serum creatinine, -2.61 versus -3.81 (mg/mL)-1 per year, p <0.001)[4]
Chemical synthesis:[5]

Tolvaptan is
chemically,
N-[4-[(7-chloro-2,3,4,5-tetrahydro-5-hydroxy1H-1-benzazepin-1-yl)carbonyl]-3-methylphenyl]-2-methylbenzamide. Tolvaptan is
represented by the following structure:
Tolvaptan, also known as OPC-41061, is a selective, competitive arginine vasopressin receptor 2 antagonist used to treat hyponatremia (low blood sodium levels) associated with congestive heart failure, cirrhosis, and the syndrome of inappropriate antidiuretic hormone (SIADH). Tolvaptan is sold by Otsuka Pharmaceutical Co. under the trade name Samsca.
Tolvaptan and its process for preparation were disclosed in U.S. Pat. No. 5,258,510.
Processes for the preparation of 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepin-5-one, 7-chloro-1-(2-methyl-4-nitrobenzoyl)-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine and 7-chloro-1-[2-methyl-4-[(2-methylbenzoyl)amino]benzoyl]-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine were reported in Bioorganic & medicinal chemistry 7 (1999), 1743-1754. According to the journal, 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepin-5-one can be prepared by reacting 7-chloro-4-ethoxycarbonyl-5-oxo-N-p-toluenesufonyl-2,3,4,5-tetrahydro-1H-1-benzazepine with acetic acid in the presence of hydrochloric acid and water to obtain 7-chloro-5-oxo-2,3,4,5-tetrahydro-1-p-toluenesulfonyl-1H-1-benzazepine, and then reacted with polyphospholic acid. According to the journal, 7-chloro-1-(2-methyl-4-nitrobenzoyl)-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine can be prepared by reacting 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine with 2-methyl-4-nitobenzoyl chloride in the presence of triethylamine.
According to the journal, 7-chloro-1-[2-methyl-4-[(2-methylbenzoyl)amino]benzoyl]-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine can be prepared by reacting 1-(4-amino-2-methylbenzoyl)-7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine with 2-methylbenzoylchloride in the presence of triethylamine.
PCT publication no. WO 2007/026971 disclosed a process for the preparation oftolvaptan can be prepared by the reduction of 7-chloro-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2,3,4,5-tetrahydro-1H-1-benzazepin-5-one with sodium borohydride.
7-Chloro-2,3,4,5-tetrahydro-1H-1-benzazepin-5-one is a key intermediate for the preparation of tolvaptan.
Biooganic and Medicinal Chemistry I (2007) 6455-6458, Biooganic andMedicinal Chemistry 14 (2000) 2493-2495 reported in the literature of the intermediate 2 - carboxylic acid -5 - (2 - methyl-benzoylamino) toluene synthesis method,

Tolvaptan, also known as OPC-41061, is a selective, competitive arginine vasopressin receptor 2 antagonist used to treat hyponatremia (low blood sodium levels) associated with congestive heart failure, cirrhosis, and the syndrome of inappropriate antidiuretic hormone (SIADH). Tolvaptan is sold by Otsuka Pharmaceutical Co. under the trade name Samsca.
Tolvaptan and its process for preparation were disclosed in U.S. Pat. No. 5,258,510.
Processes for the preparation of 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepin-5-one, 7-chloro-1-(2-methyl-4-nitrobenzoyl)-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine and 7-chloro-1-[2-methyl-4-[(2-methylbenzoyl)amino]benzoyl]-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine were reported in Bioorganic & medicinal chemistry 7 (1999), 1743-1754. According to the journal, 7-chloro-2,3,4,5-tetrahydro-1H-1-benzazepin-5-one can be prepared by reacting 7-chloro-4-ethoxycarbonyl-5-oxo-N-p-toluenesufonyl-2,3,4,5-tetrahydro-1H-1-benzazepine with acetic acid in the presence of hydrochloric acid and water to obtain 7-chloro-5-oxo-2,3,4,5-tetrahydro-1-p-toluenesulfonyl-1H-1-benzazepine, and then reacted with polyphospholic acid. According to the journal, 7-chloro-1-(2-methyl-4-nitrobenzoyl)-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine can be prepared by reacting 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine with 2-methyl-4-nitobenzoyl chloride in the presence of triethylamine.
According to the journal, 7-chloro-1-[2-methyl-4-[(2-methylbenzoyl)amino]benzoyl]-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine can be prepared by reacting 1-(4-amino-2-methylbenzoyl)-7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine with 2-methylbenzoylchloride in the presence of triethylamine.
PCT publication no. WO 2007/026971 disclosed a process for the preparation oftolvaptan can be prepared by the reduction of 7-chloro-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2,3,4,5-tetrahydro-1H-1-benzazepin-5-one with sodium borohydride.
7-Chloro-2,3,4,5-tetrahydro-1H-1-benzazepin-5-one is a key intermediate for the preparation of tolvaptan.
Biooganic and Medicinal Chemistry I (2007) 6455-6458, Biooganic andMedicinal Chemistry 14 (2000) 2493-2495 reported in the literature of the intermediate 2 - carboxylic acid -5 - (2 - methyl-benzoylamino) toluene synthesis method,


5-Chloro-2-nitrobenzoic acid (I) was converted into methyl ester (II) using dimethyl sulfate and K2CO3 in acetone. The nitro group of (II) was then reduced with SnCl2 to afford aniline (III), which was protected as the p-toluenesulfonamide (IV) with tosyl chloride in pyridine. Alkylation of (IV) with ethyl 4-bromobutyrate (V) yielded diester (VI). Subsequent Dieckmann cyclization of (VI) in the presence of potassium tert-butoxide provided benzazepinone (VIIa-b) as a mixture of ethyl and methyl esters, which was decarboxylated to (VIII) by heating with HCl in AcOH. Deprotection of the tosyl group of (VIII) was carried out in hot polyphosphoric acid. The resulting benzazepinone (IX) was condensed with 2-methyl-4-nitrobenzoyl chloride (X) to give amide (XI). After reduction of the nitro group of (XI) to the corresponding aniline (XII), condensation with 2-methylbenzoyl chloride (XIII) provided diamide (XIV). Finally, ketone reduction in (XIV) by means of NaBH4 led to the target compound.

..............................................
PATENT
CN102382053A
.....................................................
PATENT
CN102060769
Synthesis of Intermediate III: 1.
Example
2-methyl-4-nitrobenzoic acid (available from Alfa Aesar Tianjin Chemical Co., purity> 99%, 25g,
0.14mol) was added to a 250ml reaction flask, is reacted with thionyl chloride under reflux conditions for 3h, thionyl chloride was distilled off under reduced pressure to give 2-methyl-4-nitrobenzoyl chloride (26.Sg, light yellow oily liquid), without purification, was used directly in the next step.
Intermediate II (20g, 0.1moI) and 2_ methyl _4_ nitrobenzoylchloride (22.4g, 0.llmol) was added to a 250ml reaction flask. Dichloromethane (50ml), cooled to ice bath with stirring to dissolve O~5 ° C, was slowly added dropwise N- methylmorpholine (11.2g, 0.llmol), Bi dropwise with stirring while, at room temperature the reaction 4h. TLC [developing solvent: ethyl acetate - petroleum ether (I: I), hereinafter] is displayed after completion of the reaction, saturated aqueous sodium bicarbonate (20ml), stirred for lOmin, filtered, the filter cake with dichloromethane (15ml X 2 ) washing. The filtrate and washings were combined, washed with saturated sodium chloride solution (30ml X 3), dried over anhydrous sodium sulfate and filtered. The filtrate under reduced pressure to recover the solvent, the residue was recrystallized from anhydrous methanol to give a white powder 111 (27.5g, 75.2%), mp 154.8 ~155.6 ° C. Purity 97.9% (HPLC normalization method).
Synthesis of Intermediate IV:
Intermediate III (10g, 28mmol) was added to a 250ml reaction flask, concentrated hydrochloric acid (40ml) and ethanol (50ml), with stirring, was slowly added dropwise stannous chloride (20g, 88mmol) in ethanol (40ml) . Bi room temperature drops 5h. After TLC showed completion of the reaction, ethanol was distilled off under reduced pressure to about 70ml, the residue was -10 ° C -0 ° C allowed to stand overnight to cool. Filtered, and the filter cake was washed with water poured into water (40ml) in. Plus 20% sodium hydroxide solution (approximately 60ml) was adjusted to pH 9. Filtered, washed with ethanol and recrystallized to give a pale yellow powdered solid IV (6.3g, 68.7%), mp 190.4~191.1 ° C. Purity 97.2% (HPLC normalization method).
Synthesis of intermediate V:
Intermediate IV (5g, 15mmol) and triethylamine (2.3g, 23mmol) was added followed by IOOml reaction flask was added dichloromethane (30ml), stir until dissolved. Solution of o-methylbenzoyl chloride (2.8g, 18mmol), dropwise at room temperature completion of the reaction Ih0 TLC showed the reaction was complete was poured into ice-water (about 40ml) in, (20ml X 3) and extracted with dichloromethane, the combined organic phases, and saturated sodium chloride solution successively (25ml X 3), dried over anhydrous sodium sulfate and filtered with 5% hydrochloric acid (25ml X 3). The filtrate under reduced pressure to recover the solvent (about 50ml), dried over anhydrous methanol residue - petroleum ether (2: 1) and recrystallized to give white crystals of Intermediate V (6.2g, 90.9%), mp 121.1 ~123.6 ° C. Purity 98.6% (HPLC normalization method).
Synthesis of tolvaptan: Example 4
Intermediate V (5g, Ilmmol) IOOml added to the reaction flask, was added anhydrous methanol (25ml), stirred and then added portionwise sodium borohydride (0.65g, 17mmol) to the reaction mixture, addition was complete the reaction at room temperature lh. After TLC showed the reaction was complete, the methanol recovered under reduced pressure (approximately 20ml), the residue was added methylene chloride (25ml), (25mlX3) and washed with saturated sodium chloride solution. Anhydrous sodium sulfate and filtered, and the filtrate under reduced pressure to recover the solvent, the residue with absolute methanol - petroleum ether (2: 1) and recrystallized tolvaptan white crystals (4.85g, 96.6%), mp 220.1~221.5 ° C. Purity 99.2% (HPLC normalization method). ES1-HRMS (C26H25C1N203, m / z) found (calc): 447.1476 (447.1481) [MH] - "
..............
PATENT
http://www.google.com/patents/WO2012046244A1?cl=en
.....................................................
PATENT
CN102060769
Synthesis of Intermediate III: 1.
Example
2-methyl-4-nitrobenzoic acid (available from Alfa Aesar Tianjin Chemical Co., purity> 99%, 25g,
0.14mol) was added to a 250ml reaction flask, is reacted with thionyl chloride under reflux conditions for 3h, thionyl chloride was distilled off under reduced pressure to give 2-methyl-4-nitrobenzoyl chloride (26.Sg, light yellow oily liquid), without purification, was used directly in the next step.
Intermediate II (20g, 0.1moI) and 2_ methyl _4_ nitrobenzoylchloride (22.4g, 0.llmol) was added to a 250ml reaction flask. Dichloromethane (50ml), cooled to ice bath with stirring to dissolve O~5 ° C, was slowly added dropwise N- methylmorpholine (11.2g, 0.llmol), Bi dropwise with stirring while, at room temperature the reaction 4h. TLC [developing solvent: ethyl acetate - petroleum ether (I: I), hereinafter] is displayed after completion of the reaction, saturated aqueous sodium bicarbonate (20ml), stirred for lOmin, filtered, the filter cake with dichloromethane (15ml X 2 ) washing. The filtrate and washings were combined, washed with saturated sodium chloride solution (30ml X 3), dried over anhydrous sodium sulfate and filtered. The filtrate under reduced pressure to recover the solvent, the residue was recrystallized from anhydrous methanol to give a white powder 111 (27.5g, 75.2%), mp 154.8 ~155.6 ° C. Purity 97.9% (HPLC normalization method).
Synthesis of Intermediate IV:
Intermediate III (10g, 28mmol) was added to a 250ml reaction flask, concentrated hydrochloric acid (40ml) and ethanol (50ml), with stirring, was slowly added dropwise stannous chloride (20g, 88mmol) in ethanol (40ml) . Bi room temperature drops 5h. After TLC showed completion of the reaction, ethanol was distilled off under reduced pressure to about 70ml, the residue was -10 ° C -0 ° C allowed to stand overnight to cool. Filtered, and the filter cake was washed with water poured into water (40ml) in. Plus 20% sodium hydroxide solution (approximately 60ml) was adjusted to pH 9. Filtered, washed with ethanol and recrystallized to give a pale yellow powdered solid IV (6.3g, 68.7%), mp 190.4~191.1 ° C. Purity 97.2% (HPLC normalization method).
Synthesis of intermediate V:
Intermediate IV (5g, 15mmol) and triethylamine (2.3g, 23mmol) was added followed by IOOml reaction flask was added dichloromethane (30ml), stir until dissolved. Solution of o-methylbenzoyl chloride (2.8g, 18mmol), dropwise at room temperature completion of the reaction Ih0 TLC showed the reaction was complete was poured into ice-water (about 40ml) in, (20ml X 3) and extracted with dichloromethane, the combined organic phases, and saturated sodium chloride solution successively (25ml X 3), dried over anhydrous sodium sulfate and filtered with 5% hydrochloric acid (25ml X 3). The filtrate under reduced pressure to recover the solvent (about 50ml), dried over anhydrous methanol residue - petroleum ether (2: 1) and recrystallized to give white crystals of Intermediate V (6.2g, 90.9%), mp 121.1 ~123.6 ° C. Purity 98.6% (HPLC normalization method).
Synthesis of tolvaptan: Example 4
Intermediate V (5g, Ilmmol) IOOml added to the reaction flask, was added anhydrous methanol (25ml), stirred and then added portionwise sodium borohydride (0.65g, 17mmol) to the reaction mixture, addition was complete the reaction at room temperature lh. After TLC showed the reaction was complete, the methanol recovered under reduced pressure (approximately 20ml), the residue was added methylene chloride (25ml), (25mlX3) and washed with saturated sodium chloride solution. Anhydrous sodium sulfate and filtered, and the filtrate under reduced pressure to recover the solvent, the residue with absolute methanol - petroleum ether (2: 1) and recrystallized tolvaptan white crystals (4.85g, 96.6%), mp 220.1~221.5 ° C. Purity 99.2% (HPLC normalization method). ES1-HRMS (C26H25C1N203, m / z) found (calc): 447.1476 (447.1481) [MH] - "
..............
PATENT
Tolvaptan is chemically, N-[4-[(7-chloro-2,3,4,5-tetrahydro-5-hydroxylH-l- benzazepin- 1 -yl)carbonyl]-3-methylphenyl]-2-methylbenzamide. Tolvaptan is represented by the following structure:
Tolvaptan, also known as OPC-41061, is a selective, competitive arginine vasopressin receptor 2 antagonist used to treat hyponatremia (low blood sodium levels) associated with congestive heart failure, cirrhosis, and the syndrome of inappropriate antidiuretic hormone (SIADH). Tolvaptan is sold by Otsuka Pharmaceutical Co. under the trade name Samsca.
Tolvaptan and its process for preparation were disclosed in U.S. patent no. 5,258,510. Processes for the preparation of 7-chloro-2,3,4,5-tetrahydro-lH-l-benzazepin-5- one, 7-chloro-l-(2-methyl-4-nitrobenzoyl)-5-oxo-2,3,4,5-tetrahydro-lH-l-benzazepine and 7-chloro- 1 -[2-methyl-4-[(2-methylbenzoyl)amino]benzoyl]-5-oxo-2,3,4,5- tetrahydro-lH-l-benzazepine were reported in Bioorganic & medicinal chemistry 7 (1999), 1743-1754. According to the journal, 7-chloro-2,3,4,5-tetrahydro-lH-l- benzazepin-5-one can be prepared by reacting 7-chloro-4-ethoxycarbonyl-5-oxo-N-p- toluenesufonyl-2,3,4,5-tetrahydro-lH-l-benzazepine with acetic acid in the presence of hydrochloric acid and water to obtain 7-chloro-5-oxo-2,3,4,5-tetrahydro-l-p- toluenesulfonyl-lH-l-benzazepine, and then reacted with polyphospholic acid.
According to the journal, 7-chloro- 1 -(2 -methyl-4-nitrobenzoyl)-5-oxo-2,3,4,5- tetrahydro-lH-l-benzazepine can be prepared by reacting 7-chloro-5-oxo-2,3,4,5- tetrahydro-lH-l-benzazepine with 2-methyl-4-nitobenzoyl chloride in the presence of triethylamine.
According to the journal, 7-chloro- l-[2-methyl-4-[(2- methylbenzoyl)amino]benzoyl]-5-oxo-2,3,4,5-tetrahydro-lH-l-benzazepine can be prepared by reacting l-(4-amino-2-methylbenzoyl)-7-chloro-5-oxo-2,3,4,5-tetrahydro- lH-l-benzazepine with 2-methylbenzoylchloride in the presence of triethylamine.
PCT publication no. WO 2007/026971 disclosed a process for the preparation of tolvaptan can be prepared by the reduction of 7-chloro- l-[2-methyl-4-(2- methylbenzoylamino)benzoyl]-2,3,4,5-tetrahydro-lH-l-benzazepin-5-one with sodium borohydride.
7-Chloro-2,3,4,5-tetrahydro-lH-l-benzazepin-5-one is a key intermediate for the preparation of tolvaptan.

SYNTHESIS CONSTRUCTION
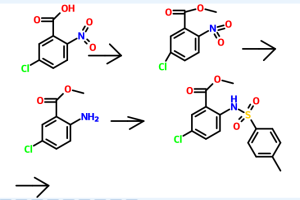



Reference example 1 :
Preparation of methyl 5-chloro-2-nitrobenzoate
Potassium carbonate (515 gm) was added to a solution of 5-chloro-2-nitro benzoic acid (500 gm) in acetone (2750 ml) at room temperature. Dimethyl sulphate (306.5 gm) was added to the reaction mixture slowly and heated to reflux for 30 minutes. The reaction mass was filtered and then concentrated to obtain a residual mass. The residual mass was poured to the ice water and extracted with methylene chloride. The solvent was distilled off under reduced pressure to obtain a residual solid of methyl 5- chloro-2-nitrobenzoate (534 gm).
Reference example 2:
Preparation of methyl 2-amino-5-chlorobenzoate
A mixture of methyl 5-chloro-2-nitrobenzoate (534 gm) as obtained in reference example 1 and concentrated hydrochloric acid (2250 ml) was added to ethyl acetate (1120 ml). To the reaction mixture was added a solution of tin chloride (1680 gm) in ethyl acetate (2250 ml). The reaction mass was stirred for 16 hours at room temperature and then poured to the ice water. The pH of the reaction mass was adjusted to 8.0 to 9.0 with aqueous sodium hydroxide solution (2650 ml). The separated aqueous layer was extracted with ethyl acetate and then concentrated to obtain a residual solid of methyl 2- amino-5-chlorobenzoate (345 gm).
Reference example 3:
Preparation of methyl 5-chIoro-2-(N-p-toluenesulfonyl)aminobenzoate
To a solution of methyl-2-amino-5-chloro benzoate (345 gm) as obtained in reference example 2 in pyridine (1725 ml) was added p-toluenesulfonyl chloride (425 gm). The reaction mixture was stirred for 2 hours at room temperature and poured to the ice water. The separated solid was filtered and dried to obtain 585 gm of methyl 5- chloro-2-(N-p-toluenesulfonyl)aminobenzoate.
Reference example 4:
Preparation of methyl 5-chloro-2-[N-(3-ethoxycarbonyI)propyI-N-p- toluenesulfonyl] aminobenzoate
Methyl 5-chloro-2-(N-p-toluenesulfonyl)aminobenzoate (585 gm) as obtained in reference example 3, ethyl-4-bromo butyrate (369.6 gm) and potassium carbonate (664 gm) in dimethylformamide (4400 ml) were added at room temperature. The contents were heated to 120°C and maintained for 2 hours. The reaction mass was poured into water and filtered. The solid obtained was dried to give 726 gm of methyl 5-chloro-2-[N- (3 -ethoxycarbonyl)propyl-N-p-toluenesulfonyl] aminobenzoate.
Reference example 5:
Preparation of 7-chloro-4-ethoxycarbonyI-5-oxo-N-p-toluenesufonyl-2,3,4,5- tetrahydro-lH-l-benzazepine
To a heated mixture of potassium tetrabutoxide (363 gm) in toluene (1000 ml) at 70°C was added portion wise methyl 5-chloro-2-[N-(3-ethoxycarbonyl)propyl-N-p- toluenesulfonyl]aminobenzoate (726 gm) as obtained in reference example 4. The contents were heated to reflux and maintained for 30 minutes. The reaction mass was then cooled to room temperature and then poured to the ice water. The layers were separated and the aqueous layer was extracted with toluene. The solvent was distilled off under reduced pressure to obtain a residual solid of 7-chloro-4-ethoxycarbonyl-5-oxo-N- p-toluenesufonyl-2,3,4,5-tetrahydro-lH-l-benzazepine (455 gm).
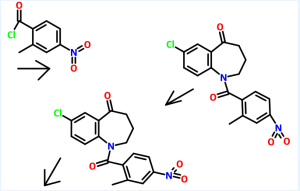
Example 1:
Preparation of 7-chIoro-5-oxo-2,3,4,5-tetrahydro-lH-l-benzazepine
7-Chloro-4-ethoxycarbonyl-5-oxo-N-p-toluenesufonyl-2,3,4,5-tetrahydro- 1 H- 1 - benzazepine (455 gm) as obtained in reference example 5 was added to aqueous sulfuric acid (80%, 2275 ml). The contents heated to 75°C and maintained for 2 hours. The reaction mass was then cooled to room temperature and then poured to the ice water. The pH of the reaction mass was adjusted to 7.5 to 8.0 with sodium hydroxide solution (2575 ml). The solid obtained was collected by filtration and dried to give 160 gm of 7- chloro-5-oxo-2,3 ,4,5-tetrahydro- 1 H- 1 -benzazepine.
Example 2:
Preparation of 7-chIoro-l-(2-methyl-4-nitrobenzoyl)-5-oxo-2,3,4,5-tetrahydro-lH-l- benzazepine
7-Chloro-5-oxo-2,3,4,5-tetrahydro-lH-l -benzazepine (160 gm) as obtained in example 1 was dissolved in methylene dichloride (480 ml) and then added aqueous sodium bicarbonate solution (20%, 68.75 gm). The reaction mixture was then cooled to 0 to 5°C and then added 2-methyl-4-nitrobenzoylchloride (180 gm) slowly. The pH of the reaction mass was adjusted to 7.0 to 8.0 with aqueous sodium bicarbonate solution (170 ml). The layers were separated and the aqueous layer was extracted with methylene chloride. The solvent was distilled off under reduced pressure to obtain a residual mass. To the residual mass was dissolved in isopropyl alcohol (7300 ml) and maintained for 2 hours at reflux temperature. The separated solid was filtered and dried to obtain 250 gm of 7-chloro-l-(2-methyl-4-nitrobenzoyl)-5-oxo-2,3,4,5-tetrahydro-lH-l-benzazepine.
Example 3:
Preparation of l-(4-amino-2-methylbenzoyl)-7-chIoro-5-oxo-2,3,4,5-tetrahydro-lH- 1-benzazepine
7-Chloro- 1 -(2-methyl-4-nitrobenzoyl)-5-oxo-2,3 ,4,5-tetrahydro- 1 H- 1 - benzazepine (250 gm) as obtained in example 2 was dissolved in methanol (575 ml) and then added a solution of tin chloride (630 gm) in methanol (1130 ml). The reaction mixture was stirred for 16 hours at room temperature and then poured to the ice water. The pH of the reaction mass was adjusted to 8.0 to 9.0 with sodium hydroxide solution (1250 ml). The layers were separated and the aqueous layer was extracted with ethyl acetate. The solvent was distilled off under vacuum to obtain a residual solid of l-(4- amino-2-methylbenzoyl)-7-chloro-5-oxo-2,3,4,5-tetrahydro- 1 H- 1 -benzazepine (185 gm).
Example 4:
Preparation of 7-chloro-l-[2-methyl-4-[(2-methylbenzoyl)amino]benzoyl]-5-dxo- 2,3,4,5-tetrahydro-lH-l-benzazepine
1 -(4-Amino-2-methylbenzoyl)-7-chloro-5-oxo-2,3 ,4,5-tetrahydro- 1 H- 1 - benzazepine (185 gm) as obtained in example 3 was dissolved in methylene chloride (4000 ml) and then added sodium bicarbonate solution (10%, 47.3 gm). The reaction mass was cooled to 0 to 5°C and then added 2-methyl benzoyl chloride (95.7 gm) slowly. -The pH of the reaction mass was adjusted to 7.0 to 8.0 with aqueous sodium bicarbonate solution (120 ml). The separated aqueous layer was extracted with methylene chloride and then concentrated to obtain a residual solid of 7-chloro-l-[2- methyl-4-[(2-methylbenzoyl)amino]benzoyl]-5-oxo-2,3,4,5-tetrahydro- 1 H- 1 - benzazepine (185 gm).
Example 5:
Preparation of tolvaptan
7-Chloro- 1 -[2-methyl-4-[(2-methylbenzoyl)amino]benzoyl]-5-oxo-2,3,4,5- tetrahydro-lH-1 -benzazepine (63 gm) as obtained in example 4 was dissolved in methanol (570 ml) and then added sodium borohydride (2.07 gm) at room temperature. The reaction mass was stirred for 1 hour and pH of the reaction mass was adjusted to 6.0 to 7.0 with hydrochloric acid solution (1%, 630 ml). The separated solid was filtered and dried to obtain 57 gm of tolvaptan.
 TOLVAPTAN
TOLVAPTAN...................
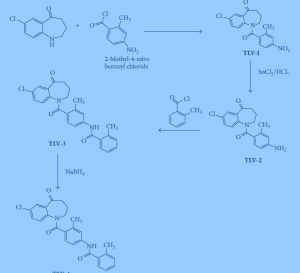 .
.
Process used to prepare Tolvaptan involves condensing 7-chloro-1, 2, 3, 4-tetrahydro-benzo[b]azepin-5-one with 2-methyl, 4-nitro benzoyl chloride, followed by reduction using SnCl2/HCl catalyst resulting in amine which is then condensed with o-toluoyl chloride followed by reduction with sodium borohydride to give Tolvaptan

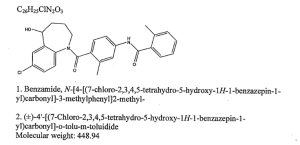

Title: Tolvaptan
CAS Registry Number: 150683-30-0
CAS Name: N-[4-[(7-Chloro-2,3,4,5-tetrahydro-5-hydroxy-1H-1-benzazepin-1-yl)carbonyl]-3-methylphenyl]-2-methylbenzamide
Additional Names: 7-chloro-5-hydroxy-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2,3,4,5-tetrahydro-1H-1-benzazepine
Manufacturers' Codes: OPC-41061
Molecular Formula: C26H25ClN2O3
Molecular Weight: 448.94
Percent Composition: C 69.56%, H 5.61%, Cl 7.90%, N 6.24%, O 10.69%
Literature References: Nonpeptide arginine vasopressin V2 receptor antagonist. Prepn: H. Ogawa et al., WO 9105549; eidem, US5258510 (1991, 1993 both to Otsuka); K. Kondo et al., Bioorg. Med. Chem. 7, 1743 (1999). Pharmacology: Y. Yamamura et al., J. Pharmacol. Exp. Ther. 287, 860 (1998). Clinical trial in heart failure: M. Gheorghiade et al., J. Am. Med. Assoc. 291, 1963 (2004).
Properties: Colorless prisms, mp 225.9°.
Melting point: mp 225.9°
Therap-Cat: In treatment of congestive heart failure.
Keywords: Vasopressin Receptor Antagonist.
- Shoaf S, Elizari M, Wang Z, et al. (2005). “Tolvaptan administration does not affect steady state amiodarone concentrations in patients with cardiac arrhythmias”. J Cardiovasc Pharmacol Ther 10 (3): 165–71. doi:10.1177/107424840501000304. PMID 16211205.
- Otsuka Maryland Research Institute, Inc.
- Gheorghiade M, Gattis W, O’Connor C, et al. (2004). “Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial”. JAMA 291 (16): 1963–71. doi:10.1001/jama.291.16.1963. PMID 15113814.
- (2012) Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease
- Kondo, K.; Ogawa, H.; Yamashita, H.; Miyamoto, H.; Tanaka, M.; Nakaya, K.; Kitano, K.; Yamamura, Y.; Nakamura, S.; Onogawa, T.; et al.; Bioor. Med. Chem. 1999, 7, 1743.
- http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm350185.htm?source=govdelivery
- Gheorghiade M, Niazi I, Ouyang J et al. (2003). “Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial”. Circulation 107 (21): 2690–6. doi:10.1161/01.CIR.0000070422.41439.04.PMID 12742979.
H. D. Zmily, S. Daifallah, and J. K. Ghali, “Tolvaptan, hyponatremia, and heart failure,” International Journal of Nephrology and Renovascular Disease, vol. 4, pp. 57–71, 2011.
M. N. Ferguson, “Novel agents for the treatment of hyponatremia: a review of conivaptan and tolvaptan,” Cardiology in Review, vol. 18, no. 6, pp. 313–321, 2010.
H. Ogawa, H. Miyamoto, K. Kondo, et al., US5258510, 1993.
K. Kondo, H. Ogawa, H. Yamashita et al., “7-Chloro-5-hydroxy-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2,3,4,5- tetrahydro-1H-1-benzazepine (OPC-41061): a potent, orally active nonpeptide arginine vasopressin V2 receptor antagonist,” Bioorganic and Medicinal Chemistry, vol. 7, no. 8, pp. 1743–1754, 1999.
| WO2012046244A1 * | Aug 23, 2011 | Apr 12, 2012 | Hetero Research Foundation | Process for preparing tolvaptan intermediates |
| CN102060769A * | Dec 20, 2010 | May 18, 2011 | 天津药物研究院 | Preparation method of tolvaptan |
| CN102060769B | Dec 20, 2010 | Sep 18, 2013 | 天津药物研究院 | Preparation method of tolvaptan |
| US9024015 | Aug 23, 2011 | May 5, 2015 | Hetero Research Foundation | Process for preparing tolvaptan intermediates |
| Cited Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| CN101817783A | May 12, 2010 | Sep 1, 2010 | 天津泰普药品科技发展有限公司 | Method for preparing tolvaptan intermediate |
| WO2007026971A2 | Sep 1, 2006 | Mar 8, 2007 | Otsuka Pharma Co Ltd | Process for preparing benzazepine compounds or salts thereof |
| Reference | ||
|---|---|---|
| 1 | Cordero-Vargas, Alejandro | |
| 2 | Kondo, Kazumi et al.7-chloro-5-hydroxy-1-[2-methyl-4-(2-methylbenzoyl-amino)benzoyl]-2,3,4,5-tetrahydro-1H-1-benzazepine (OPC-41061): A potent, orally active nonpeptide arginine vasopressin V2 receptor antagonist.《Bioorganic & Medicinal Chemistry》.1999,1743-1757. | |
| 3 | Quiclet-Sire, Beatrice | |
| 4 | Torisawa, Yasuhiro et al.Aminocarbonylation route to tolvaptan.《Bioorganic & Medicinal Chemistry Letters》.2007,6455-6458. | |
| 5 | Zard,
Samir Z.A flexible approach for the preparation of substituted
benzazepines: Application to the synthesis of tolvaptan.《Bioorganic
& Medicinal Chemistry》.2006,6165-6173. सुकून उतना ही देना प्रभू, जितने से जिंदगी चल जाये। औकात बस इतनी देना, कि औरों का भला हो जाये।  LIONEL MY SON LIONEL MY SON
He was only in first standard in school when I was hit by a deadly one in a million spine stroke called acute transverse mylitis, it made me 90% paralysed and bound to a wheel chair, Now I keep him as my source of inspiration and helping millions, thanks to millions of my readers who keep me going and help me to keep my son happy
जिंदगी चल जाये। औकात बस इतनी देना, कि औरों का भला हो जाये। Read all about Organic Spectroscopy on ORGANIC SPECTROSCOPY INTERNATIONAL | |




MDL 29913 acts as selective antagonist of NK2 tachykinin receptor. It is used for the treatment of IBS. MDL 29,913
ReplyDelete