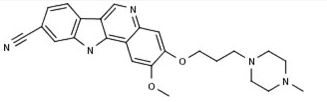
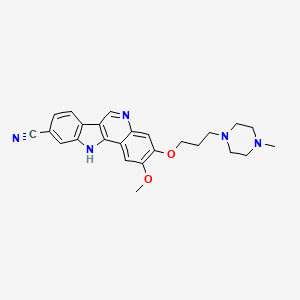
PF-05387252
CAS 1604034-71-0
| C25H27N5O2 | |
| MW | 429.51418 g/mol |
|---|
IRAK4 inhibitor
Rheumatoid arthritis;
SLE
Preclinical
In
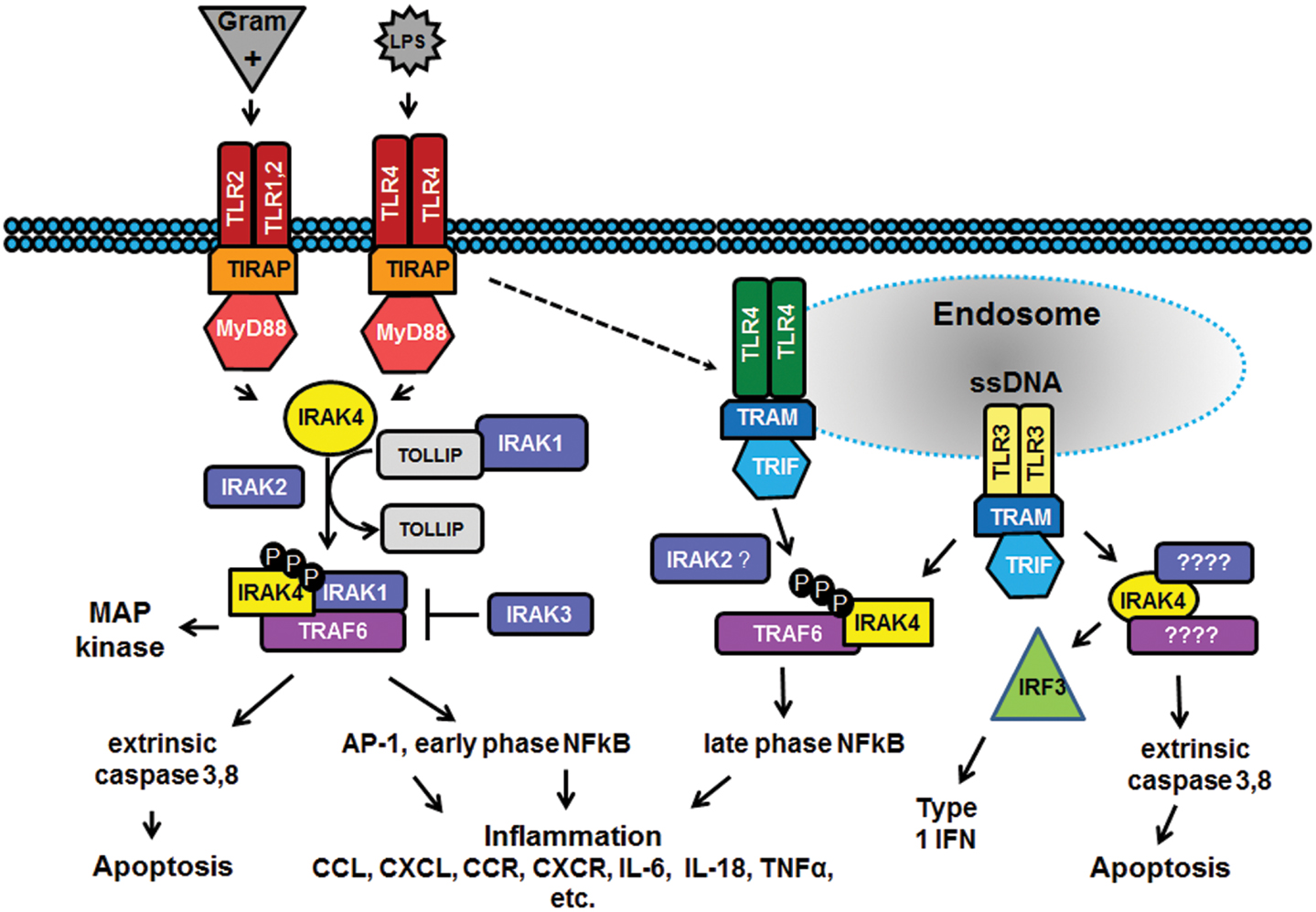
the past decade there has been considerable interest in targeting the
innate immune system in the treatment of autoimmune diseases and sterile
inflammation. Receptors of the innate immune system provide the first
line of defense against bacterial and viral insults. These receptors
recognize bacterial and viral products as well as pro-inflammatory
cytokines and thereby initiate a signaling cascade that ultimately
results in the up-regulation of inflammatory cytokines such as TNFα,
IL6, and interferons. Recently it has become apparent that
self-generated ligands such as nucleic acids and products of
inflammation such as HMGB1 and Advanced Glycated End-products (AGE) are
ligands for Toll-like receptors (TLRs) which are key receptors of the
innate immune system.
This demonstrates the role of TLRs in the initiation and perpetuation of inflammation due to autoimmunity.
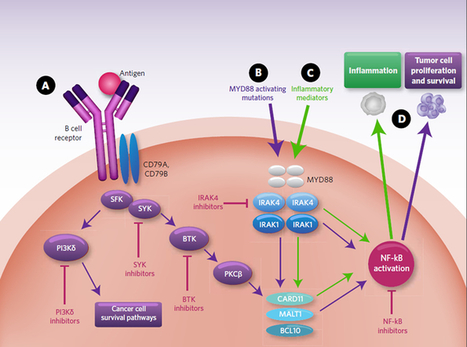
Interleukin-1
receptor associated kinase (IRAK4) is a ubiquitously expressed
serine/threonine kinase involved in the regulation of innate
immunity. IRAK4 is responsible for initiating signaling from TLRs and
members of the IL-1/18 receptor family. Kinase-inactive knock-ins and
targeted deletions of IRAK4 in mice lead to reductions in TLR and IL-1
induced pro-inflammatory cytokines. and 7
IRAK-4 kinase-dead knock-in mice have been shown to be resistant to
induced joint inflammation in the antigen-induced-arthritis (AIA) and
serum transfer-induced (K/BxN) arthritis models. Likewise, humans
deficient in IRAK4 also display the inability to respond to challenge by
TLR ligands and IL-1
However,
the immunodeficient phenotype of IRAK4-null individuals is narrowly
restricted to challenge by gram positive bacteria, but not gram negative
bacteria, viruses or fungi. This gram positive sensitivity also lessens
with age implying redundant or compensatory mechanisms for innate
immunity in the absence of IRAK4.These data suggest that inhibitors
of IRAK4 kinase activity will have therapeutic value in treating
cytokine driven autoimmune diseases while having minimal
immunosuppressive side effects. Additional recent studies suggest
that targeting IRAK4 may be a viable strategy for the treatment of other
inflammatory pathologies such as atherosclerosis.
Indeed,
the therapeutic potential of IRAK4 inhibitors has been recognized by
others within the drug-discovery community as evidenced by the variety
of IRAK4 inhibitors have been reported to-date.12, 13, 14, 15 and 16
However, limited data has been published about these compounds and they
appear to suffer from a variety of issues such as poor kinase
selectivity and poor whole-blood potency that preclude their advancement
into the pre-clinical models. To the best of our knowledge, no in vivo
studies of IRAK4 inhibitors have been reported to-date in the
literature. Herein we report a new class of IRAK4 inhibitors that are
shown to recapitulate the phenotype observed in IRAK4 knockout and
kinase-dead mice.
PAPER
Bioorganic & Medicinal Chemistry Letters (2014), 24(9), 2066-2072.
doi:10.1016/j.bmcl.2014.03.056
http://www.sciencedirect.com/science/article/pii/S0960894X14002832
Identification and optimization of indolo[2,3-c]quinoline inhibitors of IRAK4
- a Pfizer Global R&D, 445 Eastern Point Rd., Groton, CT 06340, USA
- b Pfizer Global R&D, 200 Cambridge Park Dr., Cambridge, MA 02140, USA
- c Pfizer Global R&D, 87 Cambridgepark Dr., Cambridge, MA 02140, USA
- d Pfizer Global R&D, 1 Burtt Rd., Andover, MA 01810, USA


Abstract
IRAK4
is responsible for initiating signaling from Toll-like receptors (TLRs)
and members of the IL-1/18 receptor family. Kinase-inactive knock-ins
and targeted deletions of IRAK4 in mice cause reductions in TLR induced
pro-inflammatory cytokines and these mice are resistant to various
models of arthritis. Herein we report the identification and
optimization of a series of potent IRAK4 inhibitors. Representative
examples from this series showed excellent selectivity over a panel of
kinases, including the kinases known to play a role in TLR-mediated
signaling. The compounds exhibited low nM potency in LPS- and
R848-induced cytokine assays indicating that they are blocking the TLR
signaling pathway. A key compound (26)
from this series was profiled in more detail and found to have an
excellent pharmaceutical profile as measured by predictive assays such
as microsomal stability, TPSA, solubility, and c log P.
However, this compound was found to afford poor exposure in mouse upon
IP or IV administration. We found that removal of the ionizable
solubilizing group (32) led to increased exposure, presumably due to increased permeability. Compounds 26 and 32,
when dosed to plasma levels corresponding to ex vivo whole blood
potency, were shown to inhibit LPS-induced TNFα in an in vivo murine
model. To our knowledge, this is the first published in vivo
demonstration that inhibition of the IRAK4 pathway by a small molecule
can recapitulate the phenotype of IRAK4 knockout mice.


SYNTHESIS

////////PF-05387252, 1604034-71-0, PF 05387252, TLR signaling, Indoloquinoline, IRAK4, Kinase inhibitor, Inflammation, PRECLINICAL
N1(CCN(CC1)CCCOc3c(cc2c4nc5cc(ccc5c4cnc2c3)C#N)OC)C
OR
CN1CCN(CC1)CCCOC2=C(C=C3C(=C2)N=CC4=C3NC5=C4C=CC(=C5)C#N)OC
No comments:
Post a Comment