
PF 6282999
Alternative Names: PF-06282999; PF-6282999, PF-06282999
Cas 1435467-37-0
[2-(6-(5-chloro-2-methoxyphenyl)-4-oxo-2-thioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamide]
2-(6-(5-chloro-2-methoxyphenyl)-4-oxo-2-thioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamide
MF C13H12ClN3O3S
Molecular Weight: 325.767
Elemental Analysis: C, 47.93; H, 3.71; Cl, 10.88; N, 12.90; O, 14.73; S, 9.84
Molecular Weight: 325.767
Elemental Analysis: C, 47.93; H, 3.71; Cl, 10.88; N, 12.90; O, 14.73; S, 9.84
Irreversible inactivator of myeloperoxidase
Currently in clinical trials for the potential treatment of cardiovascular diseases.
Phase I
- Phase I Acute coronary syndromes
Most Recent Events
- 01 Mar 2015 Pfizer terminates phase I trial in Healthy volunteers in USA (NCT01965600)
- 10 Sep 2014 Pfizer completes enrolment in its phase I trial in Healthy volunteers in USA (NCT01965600)
- 01 Feb 2014 Phase-I clinical trials in volunteers in USA (PO)
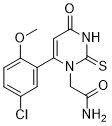

PF-06282999
is a potent and selective myeloperoxidase Inhibitor which is potential
useful for the Treatment of Cardiovascular Diseases. PF-06282999
displayed excellent oral pharmacokinetics in preclinical species and
robust irreversible inhibition of plasma MPO activity both in human
blood stimulated exogenously and in plasma collected after oral (po)
administration to lipopolysaccharide (LPS)-treated cynomolgus monkeys.
PF-06282999
has been advanced into first-in-human pharmacokinetics and safety
studies. Myeloperoxidase (MPO) is a heme peroxidase that catalyzes the
production of hypochlorous acid. Clinical evidence suggests a causal
role for MPO in various autoimmune and inflammatory disorders including
vasculitis and cardiovascular and Parkinson's diseases, implying that
MPO inhibitors may represent a therapeutic treatment option
The
thiouracil derivative PF-06282999
[2-(6-(5-chloro-2-methoxyphenyl)-4-oxo-2-thioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamide]
is an irreversible inactivator of myeloperoxidase and is currently in
clinical trials for the potential treatment of cardiovascular diseases.
Concerns over idiosyncratic toxicity arising from bioactivation of the
thiouracil motif to reactive species in the liver have been largely
mitigated through the physicochemical (molecular weight, lipophilicity,
and topological polar surface area) characteristics of PF-06282999,
which generally favor elimination via nonmetabolic routes.

To
test this hypothesis, pharmacokinetics and disposition studies were
initiated with PF-06282999 using animals and in vitro assays, with the
ultimate goal of predicting human pharmacokinetics and elimination
mechanisms. Consistent with its physicochemical properties, PF-06282999
was resistant to metabolic turnover from liver microsomes and
hepatocytes from animals and humans and was devoid of cytochrome P450
inhibition. In vitro transport studies suggested moderate intestinal
permeability and minimal transporter-mediated hepatobiliary disposition.
PF-06282999 demonstrated moderate plasma protein binding across all of
the species.
Pharmacokinetics
in preclinical species characterized by low to moderate plasma
clearances, good oral bioavailability at 3- to 5-mg/kg doses, and renal
clearance as the projected major clearance mechanism in humans. Human
pharmacokinetic predictions using single-species scaling of dog and/or
monkey pharmacokinetics were consistent with the parameters observed in
the first-in-human study, conducted in healthy volunteers at a dose
range of 20-200 mg PF-06282999.
In
summary, disposition characteristics of PF-06282999 were relatively
similar across preclinical species and humans, with renal excretion of
the unchanged parent emerging as the principal clearance mechanism in
humans, which was anticipated based on its physicochemical properties
and supported by preclinical studies.
PAPER
Journal of Medicinal Chemistry (2015), 58(21), 8513-8528.http://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.5b00963
Discovery of 2-(6-(5-Chloro-2-methoxyphenyl)-4-oxo-2-thioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamide (PF-06282999): A Highly Selective Mechanism-Based Myeloperoxidase Inhibitor for the Treatment of Cardiovascular Diseases
Worldwide Research and Development, Pfizer, Inc., Groton, Connecticut 06340, United States
J. Med. Chem., 2015, 58 (21), pp 8513–8528
DOI: 10.1021/acs.jmedchem.5b00963

Myeloperoxidase
(MPO) is a heme peroxidase that catalyzes the production of
hypochlorous acid. Clinical evidence suggests a causal role for MPO in
various autoimmune and inflammatory disorders including vasculitis and
cardiovascular and Parkinson’s diseases, implying that MPO inhibitors
may represent a therapeutic treatment option. Herein, we present the
design, synthesis, and preclinical evaluation of N1-substituted-6-arylthiouracils
as potent and selective inhibitors of MPO. Inhibition proceeded in a
time-dependent manner by a covalent, irreversible mechanism, which was
dependent upon MPO catalysis, consistent with mechanism-based
inactivation. N1-Substituted-6-arylthiouracils exhibited low
partition ratios and high selectivity for MPO over thyroid peroxidase
and cytochrome P450 isoforms. N1-Substituted-6-arylthiouracils
also demonstrated inhibition of MPO activity in
lipopolysaccharide-stimulated human whole blood. Robust inhibition of
plasma MPO activity was demonstrated with the lead compound
2-(6-(5-chloro-2-methoxyphenyl)-4-oxo-2-thioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamide (PF-06282999, 8)
upon oral administration to lipopolysaccharide-treated cynomolgus
monkeys. On the basis of its pharmacological and pharmacokinetic
profile, PF-06282999 has been advanced to first-in-human pharmacokinetic
and safety studies.
tan solid (mp = 165.3 °C).
1H NMR (500 MHz, DMSO-d6) δ 12.85 (s, 1 H), 7.57 (dd, J = 9.03, 2.68 Hz, 1 H), 7.33 (s, 1 H), 7.17–7.23 (m, 2 H), 7.10 (s, 1 H), 5.89 (d, J = 1.71 Hz, 1 H), 5.41 (br s, 1 H), 3.89 (br s, 1 H), 3.84 (s, 3 H).
MS (ES+) m/z: 326.0 [M + H]+. HRMS: m/z calcd for C13H13ClN3O3S [M + H]+ 326.0366, found 326.0361.
Anal. Calcd for C13H12ClN3O3S: C, 47.93; H, 3.71; N, 12.90; S, 9.84. Found: C, 47.81; H, 3.70; N, 12.83; S, 9.83. HPLC purity: >95%.
PATENT
WO 2013068875http://www.google.co.in/patents/WO2013068875A1?cl=en
Beta Keto Ester Route Section
A. Carboxylic Acid Route Section
Preparation 1
Ethyl 3-(5-chloro-2-methoxyphenyl)-3-oxopropanoate
A 3000 mL 3-necked round-bottomed flask flushed with nitrogen was charged with magnesium ethoxide (67.46 g, 589.51 mmoles) and THF (1 100 mL), and the resulting mixture was stirred as ethyl hydrogen malonate (162.26 g, 1 .18 moles; 145.00 mL diluted in 100 ml of THF) was added and the mixture was heated at 45 °C for 4 hours. Meanwhile, a 2000 mL 3-necked round-bottomed flask flushed with nitrogen was charged with 5-chloro-2-methoxybenzoic acid (100 g, 536 mmoles) and THF (600 mL). To this mixture stirring at room temperature was added 1 , 1 '-carbonyldiimidazole (95.59 g, 589.5 mmoles) in portions to avoid excess foaming. After stirring for 3 hours at room temperature the second solution was added gradually to the first solution. After addition the reaction mixture was heated to 45 °C. After 20 hours, the reaction mixture was concentrated under reduced pressure before adding ethyl acetate (1 L) followed by 2 N HCI (500 mL). After mixing, the layers were separated and the organic phase was washed sequentially with 2 N HCI (500 mL), saturated sodium bicarbonate (500 mL), and water (500 mL). The organic phase was concentrated under reduced pressure, the residue taken up in ethyl acetate (1000 mL) and concentrated again to afford the title compound (104.94 g).
MS (ES+) 257.2 [M+1 ]+. 1 H NMR showed product as a 7.5:1 keto:enol mixture. For the keto tautomer: 1 H NMR (500 MHz, CDCI3) δ ppm 7.85 (d, J=2.93 Hz, 1 H) 7.45 (dd, J=8.90, 2.81 Hz, 1 H) 6.92 (d, J=8.78 Hz, 1 H) 4.18 (q, J=7.16 Hz, 2 H) 3.95 (s, 2 H) 3.90 (s, 3 H) 1 .24 (t, J=7.07 Hz, 3 H). Preparation 2
(Z)-Ethyl 3-((2-amino-2-oxoethyl)amino)-3-(5-chloro-2-methoxyphenyl)acrylate A 5-L reaction vessel was charged with methanol (3.3 L), sodium methoxide (102.4 g, 1.8 moles), and glycinamide hydrochloride (202 g, 1.8 moles). The mixture was heated at 65 °C for 1 hour before cooling to 50 °C and adding acetic acid (514.25 mmoles, 30.88 g, 29.47 ml.) and ethyl 3-(5-chloro-2-methoxyphenyl)-3-oxopropanoate (300 g, 1.03 mole). After heating to reflux for 16 hours, the reaction mixture was stirred as it was cooled to 10 °C. After 30 min the resulting solid was collected by vacuum filtration, pulling dry to form a cake that was dried in a vacuum oven (20 mm Hg, 65 °C) for 14 hours to afford the title compound (339.4 g).
MS (ES+) 313.2 [M+1]+. 1H NMR (500 MHz, DMSO-d6) δ ppm 8.80 (t, J=5.00 Hz, 1 H) 7.47 (dd, J=8.90, 2.81 Hz, 1 H) 7.27 (br. s., 1 H) 7.22 (d, J=2.68 Hz, 1 H) 7.14 (d, J=8.78 Hz, 1 H) 7.09 (br. s., 1 H) 4.30 (s, 1 H) 4.03 (q, J=7.07 Hz, 2 H) 3.80 (s, 3 H) 3.56 (br. s., 1 H) 3.45 (br. s., 1 H) 1.18 (t, J=7.07 Hz, 3 H).
Example 1
2-( 6-(5-Chloro-2-methoxyphenyl)-4-oxo-2-thioxo-3, 4-dihydropyrimidin
acetamide
A reaction vessel equipped with an efficient stirrer was charged with (Z)-ethyl 3-((2- amino-2-oxoethyl)amino)-3-(5-chloro-2-methoxyphenyl)acrylate (15 g, 50.2 mmol), butyl acetate (150 ml.) and trimethylsilyl isothiocyanate (160.7 mmole, 21 .1 g, 22.7 ml.) and the mixture was heated to reflux. After 15 hours, the mixture was cooled to 30 °C and treated with 1 N aqueous sodium hydroxide (1 12.5 ml_, 1 12.5 mmoles). After 30 min, the organic layer was separated and extracted with another portion of 1 N sodium hydroxide (37.5 ml_, 37.5 mmoles). The combined aqueous phases were extracted twice with dichloromethane (2 x 45 mL), filtered, and treated with 6N HCI until a pH of 2.5 was achieved. After stirring for 1 hour, the resulting solid was isolated by vacuum filtration, resuspended in 100 mL of a 1 :1 methanol-water solution, heated with stirring at 50 °C for 2 hours, and cooled to room temperature before collecting the solid by vacuum filtration, pulling dry and drying in a vacuum oven (20 mm Hg, 50 °C) for 12 hours to afford 8.7 g of the desired product as a tan solid.
MS (ES+) 326.0 [M+1]+. 1H NMR (500 MHz, DMSO-d6) δ ppm 12.85 (s, 1 H) 7.57 (dd, J=9.03, 2.68 Hz, 1 H) 7.33 (s, 1 H) 7.17 - 7.23 (m, 2 H) 7.10 (s, 1 H) 5.89 (d, J=1.71 Hz, 1 H) 5.41 (br. s, 1 H) 3.89 (br. s, 1 H) 3.84 (s, 3 H).
Alternative Preparation of Example 1
2-( 6-( 5-Chloro-2-methoxyphenyl)-4-oxo-2-thioxo-3, 4-dihydropyrimidin- 1 ( 2H)-yl) acetamide A slurry of (Z)-ethyl 3-((2-amino-2-oxoethyl)amino)-3-(5-chloro-2- methoxyphenyl)acrylate (20 g, 63 mmol) in a mixture of butyl acetate (140 mL) and DMF (38 mL) was treated with trimethylsilyl isothiocyanate (16.8 g, 125 mmol) and the mixture was heated at 1 15-120 °C for 5-6 hours. The mixture was cooled to 0-5 °C, butyl acetate (100 mL) was added and the mixture was slurried for 8 hours. The formed solids were filtered, and the filter cake was washed with butyl acetate (2 x 100 mL). The solid was dried in a vacuum oven at 50 °C for 12 hours to a tan solid. The solid was dissolved in a 5:1 mixture of DMF and water at room temperature and additional water was added slowly to crystallize the material. The slurry was cooled to 10 °C and stirred for 8 hours, followed by filtration and washing with water. The filter cake was dried in a vacuum oven at 50 °C for 8 hours. The solid was dissolved in a 1 :1 mixture of methanol and water and the slurry was heated to 50 °C and held at this temperature for 2 hours. After cooling to 10 °C over 30 minutes, the slurry was held at this temperature for 1 hour, filtered and washed with water and dried in a vacuum oven at 50 °C for 8 hours to give the title compound as a white solid. MS (ES+) 326.0 [M+1]+.1H NMR (500 MHz, DMSO-d6) δ ppm 12.85 (s, 1 H) 7.57 (dd, J=9.03, 2.68 Hz, 1 H) 7.33 (s, 1 H) 7.17 - 7.23 (m, 2 H) 7.10 (s, 1 H) 5.89 (d, J=1.71 Hz, 1 H) 5.41 (br. s, 1 H) 3.89 (br. s, 1 H) 3.84 (s, 3 H).
REFERENCES
1: Ruggeri RB, Buckbinder L, Bagley SW, Carpino PA, Conn EL, Dowling MS, Fernando DP, Jiao W, Kung DW, Orr ST, Qi Y, Rocke BN, Smith A, Warmus JS, Zhang Y, Bowles D, Widlicka DW, Eng H, Ryder T, Sharma R, Wolford A, Okerberg C, Walters K, Maurer TS, Zhang Y, Bonin PD, Spath SN, Xing G, Hepworth D, Ahn K, Kalgutkar AS. Discovery of 2-(6-(5-Chloro-2-methoxyphenyl)-4-oxo-2-thioxo-3,4-dihydropyrimidin-1(2H)-yl)acetamide (PF-06282999): A Highly Selective Mechanism-Based Myeloperoxidase Inhibitor for the Treatment of Cardiovascular Diseases. J Med Chem. 2015 Oct 28. [Epubahead of print] PubMed PMID: 26509551.
////////////PF 06282999, 1435467-37-0, PFIZER, PHASE 1, PF-06282999; PF-6282999, PF06282999, ACUTE CORONARY SYNDROME
O=C(N)CN(C(N1)=S)C(C2=CC(Cl)=CC=C2OC)=CC1=O



No comments:
Post a Comment