H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2

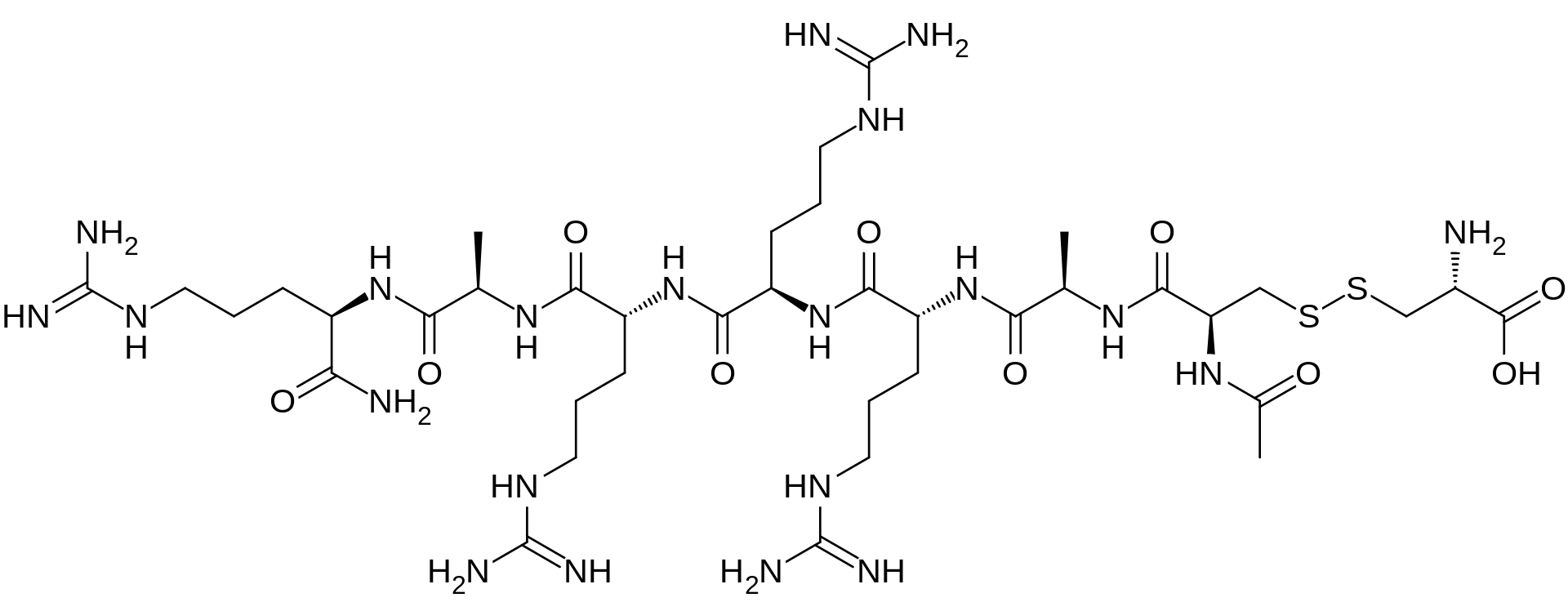
AMG 416 IS (Ac-D-Cys(L-Cys-OH)-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2)
WO 2011/014707. , the compound may be represented as follows:
H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2
The main chain has 7 amino acids, all in the D-configuration and the side-chain cysteine residue is in the L-configuration. The amino terminal is acetylated and the carboxyl-terminal is amidated. This compound ("AMG-416") has utility for the treatment of secondary hyperparathyroidism (SHPT) in hemodialysis patients. A liquid formulation comprising AMG-416 may be administered to a subject intravenously. The hydrochloride salt of AMG-416 may be represented as follows:
H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2 · x(HCl)
Therapeutic peptides pose a number of challenges with respect to their formulation. Peptides in general, and particularly those that contain a disulfide bond, typically have only moderate or poor stability in aqueous solution. Peptides are prone to amide bond hydrolysis at both high and low pH.
Disulfide bonds can be unstable even under quite mild conditions (close to neutral pH). In addition, disulfide containing peptides that are not cyclic are particularly prone to dimer formation. Accordingly, therapeutic peptides are often provided in lyophilized form, as a dry powder or cake, for later reconstitution.
A lyophilized formulation of a therapeutic peptide has the advantage of providing stability for long periods of time, but is less convenient to use as it requires the addition of one or more diluents and there is the potential risk for errors due to the use of an improper type or amount of diluent, as well as risk of contamination. In addition, the lyophilization process is time consuming and costly.
H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2
Generic Name:Etelcalcetide
Synonym:KAI-4169
CAS Number:1262780-97-1
N-acetyl-D-cysteinyl-S-(L-cysteine disulfide)-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-D-argininamide
Mechanism of Action:Activates calcium sensing receptor on parathyroid glands reducing PTH synthesis and secretion
Indication: secondary hyperparathyroidism associated with chronic kidney disease
Development Stage: Phase III
Developer:KAI Pharmaceuticals/Amgen Inc.
H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2 · x(HCl)

HYDROCHLORIDE
Generic Name:Etelcalcetide Hydrochloride
AMG 416, KAI-4169, previously also known as velcalcetide hydrochloride
CAS :1334237-71-6
Chemical Name:N-acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-D-argininamide disulfide with L-cysteine hydrochloride
Mechanism of Action:Activates calcium sensing receptor on parathyroid glands reducing PTH synthesis and secretion
Indication: secondary hyperparathyroidism associated with chronic kidney disease
Development Stage: Phase III
Developer:KAI Pharmaceuticals/Amgen Inc.
Method for preparing etelcalcetide and its salts, particularly hydrochloride. See WO2014210489, for a prior filing claiming stable liquid formulation of etelcalcetide. Amgen, following its acquisition of KAI Pharmaceuticals, and Japanese licensee Ono Pharmaceuticals are developing etelcalcetide, a long-acting iv isozyme-selective peptide-based protein kinase C epsilon inhibitor and agonist of the calcium-sensing receptor, for treating secondary hyperparathyroidism (SHPT) in patients with end-stage renal disease receiving dialysis.
In August 2015, an NDA was submitted seeking approval of the drug for SHPT in patients with chronic kidney disease (CKD) on hemodialysis (HD) in the US.
In September 2015, Amgen filed an MAA under the centralized procedure in the EU for the approval of etelcalcetide for treating SHPT in patients with CKD on HD therapy.
KAI is also investigating a transdermal patch formulation of the drug for treating primary HPT.
WO2011014707
http://www.google.com/patents/WO2011014707A2?cl=en
https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015154031&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=PCTDescription
The hydrochloride salt of AMG 416 has the chemical structure:
H-L-Cys-OH
I
s— s
I
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2 · x(HCl)
(SEQ ID NO:l)
The main chain has 7 amino acids, all in the D-configuration. The side-chain cysteine residue is in the L-configuration. The molecular formula of AMG 416 (free base) is C38H73N21O10S2, and has a calculated average molecular mass of 1048.3 Da.
AMG 416 and a method for its preparation are described in International Pat. Publication No. WO 2011/014707, which is incorporated herein by reference for any purpose. As described in International Pat. Publication No. WO 2011/014707, AMG 416 may be assembled by solid-phase synthesis from the corresponding Fmoc-protected D-amino acids. After cleavage from the resin, the material may be treated with Boc-L-Cys(NPyS)-OH to form the disulfide bond. The Boc group may then be removed with trifluoroacetate (TFA) and the resulting product purified by reverse-phase high pressure liquid chromatography (HPLC) and isolated as the TFA salt form by lyophilization. The TFA salt can be converted to a pharmaceutically acceptable salt by carrying out a subsequent salt exchange procedure. Such procedures are well known in the art and include, e.g., an ion exchange technique, optionally followed by purification of the resultant product (for example by reverse phase liquid chromatography or reverse osmosis).
There is a need for an efficient method of producing AMG 416, or a pharmaceutically acceptable salt thereof (e.g., AMG 416 HC1), and particularly one appropriate for commercial scale manufacturing.
In a first aspect, provided is a method for preparing AMG 416, the method comprising: providing a resin-bound peptide having a structure selected from the group consisting of Fmoc-D-Cys(Trt)-D-Ala-D- Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-[Resin] (SEQ ID NO:2) and Ac-D-Cys(Trt)-D-Ala-D- Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-[Resin] (SEQ ID NO:3); cleaving the peptide from the solid support; and activating the side chain of the D-Cys residue of the cleaved peptide.
In a second aspect, provided is a method for preparing AMG 416, the method comprising: providing a peptide having a structure of Ac-D-Cys(SPy)-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2 (SEQ ID NO:4); and contacting the peptide with L-Cys to produce a conjugated product.
In yet a third aspect provided is a method for preparing AMG 416, the method comprising: providing a resin-bound peptide having a structure selected from the group consisting of Fmoc-D-Cys(Trt)-D-Ala-D-Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-[Resin] (SEQ ID NO:2) and Ac-D-Cys(Trt)-D-Ala-D-Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-[Resin] (SEQ ID NO:3); cleaving the peptide from the solid support, i.e., to provide an unsupported peptide, and activating the side chain of the D-Cys residue of the unsupported peptide to generate an AMG 416 SPy intermediate (where SPy is 2-pyridinesulfenyl or S-Pyr), dissolving the AMG 416 SPy intermediate in an aqueous 0.1% TFA (trifluoroacetic acid solution), and purifying the AMG 416 SPy derivative by HPLC.
The term "AMG 416", also known as etelcalcetide, formerly known as velcalcetide or KAI-4169, refers to a compound having the chemical name: N-acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-D-arginamide disulfide with L-cysteine, which has the following structural formula:
H-L-Cys-OH
I
s— s
I
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2
Reference to AMG 416, or to any compound or AMG 416 fragment, intermediate, or precursor as described herein, is intended to encompass neutral, uncharged forms thereof, as well as pharmaceutically acceptable salts, hydrates and solvates thereof.
The terms "AMG 416 hydrochloride" and "AMG 416 HC1" are interchangeable and refer to a hydrochloride salt form of AMG 416 having the following structural formula:
H-L-Cys-OH
I
s— s
I
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2 · xHCl
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 shows the chemical structure of AMG 416 (Ac-D-Cys(L-Cys-OH)-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2) (SEQ ID NO: l).


FIG. 2 shows the chemical structure of Rink Amide AM resin and Ac-D-Cys(Trt)- D-Ala-D-Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-Resin (SEQ ID NO:3).

FIG. 3 shows a reaction scheme in which the SPy intermediate product (Ac-D-Cys(SPy)-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2) (SEQ ID NO:4) is formed from the peptidyl-resin (Ac-D-Cys(Trt)-D-Ala-D-Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-NH-Resin) (SEQ ID NO:3).

FIG. 4 shows a reaction scheme in which a TFA salt of AMG 416 is formed from the SPy intermediate (AA1_7(SPy)).

FIG. 5 shows a reaction scheme in which the HC1 salt of AMG 416 is formed from the TFA salt of AMG 416.

FIG. 6 shows a reaction scheme in which Boc-D-Arg(Pbf)-OH is formed from Boc-D-Arg-OH.

FIG. 7 shows a reaction scheme in which D-Arg(Pbf)-OH is formed from Boc-D-Arg(Pbf)-OH.

EXAMPLE 5
Purification of the SPy Intermediate and Production of AMG 416 HC1
An alternative method for preparation of AMG 416 HC1 salt is described here. As described in Example 2 above, the SPy intermediate product was dried at 20°C under full vacuum after cleavage from the resin, precipitation and filtration. The precipitate was then dissolved in a 0.1% TFA aqueous solution and loaded onto a C-18 column for HPLC purification. The column was run at <60 bar and the solution temperature was 15-25 °C throughout. The eluents were 0.1% TFA in acetonitrile and 0.1% TFA in water. The fractions were stored at 5°C, they were sampled and then fractions were pooled. The combined pools from two runs were diluted and a concentration/purification run was performed using the same HPLC column to decrease the total volume and remove additional impurities. The fractions were stored at 5°C.
The fractions containing the AMG 416 SPy intermediate were subjected to azeotropic distillation to change the solvent from the 0.1% TFA to a 15% water in IPA solution, charging with IPA as needed. To the resultant AMG 416 SPy intermediate in IPA solution was then added L-Cysteine 1.15 eq and the reaction was allowed to proceed at room temperature for conjugation to occur and to form the AMG 416 TFA salt as described above in Example 4. The AMG 416 TFA solution was added to a solution of 12M aqueous HC1, 0.27 L/kg and IPA 49.4 L/kg over 3 hours via subsurface addition, resulting in direct precipitation of the AMG 416 4.5 HC1 salt. The batch was aged for 3 hours and sampled for analysis.
The material was filtered and slurry washed with 96 wt% IPA, 10 L/kg. The cake was then re-slurried for 4 hours in 10 L/kg of 96% wt% IPA. The material was filtered and further slurry washed with 96% IPA, 10 L/kg and then IPA 10 L/kg. The material was dried under full vacuum at 25°C. The dry cake was dissolved in water 8 L/kg and the batch was concentrated via distillation to remove residual IPA and achieve the desired concentration. The solution temperature was kept below 25 °C throughout the distillation.
SEE
https://patentscope.wipo.int/search/en/detail.jsf;jsessionid=2A32CFD9CE075079399E9DD298899C9D.wapp2nC?docId=WO2014210489&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=PCTDescription
EXAMPLE 1
Solubility of AMG 416 in Succinate Buffered Saline
In this study, the solubility of AMG 416 in succinate buffered-saline was investigated. AMG 416 HC1 (103 mg powder, 80 mg peptide) was dissolved in 200 iL of sodium succinate buffered saline (25 mM succinate, 0.9% saline, pH 4.5). After briefly vortexing, a clear solution was obtained with a nominal concentration of 400 mg/mL. Because expansion of the solution volume was not determined, the solubility of AMG 416 can be conservatively stated as at least 200 mg/mL. Although the maximal solubility was not determined in this experiment, AMG 416 is soluble in pH 4.5 succinate buffered saline to concentrations of at least 200 mg/mL.
49th Congr Eur Renal Assoc - Eur Dialysis Transpl Assoc (May 24-27, Paris) 2012, Abst SAO054
//////////////Etelcalcetide, AMG 416, KAI-4169, velcalcetide
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2



AMG 416 IS (Ac-D-Cys(L-Cys-OH)-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2)
Etelcalcetide (AMG 416, KAI-4169, velcalcetide)
The main chain has 7 amino acids, all in the D-configuration. The side-chain cysteine residue is in the L-configuration. The molecular formula of AMG 416 (free base) is C38H73N21O10S2, and has a calculated average molecular mass of 1048.3 Da.D-Argininamide, N-acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-, disulfide with L-cysteine, hydrochloride (1:?)
N-Acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-D-argininamide disulfide with L-cysteine hydrochloride
http://www.amgenpipeline.com/pipeline/WO 2011/014707. , the compound may be represented as follows:
H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2
The main chain has 7 amino acids, all in the D-configuration and the side-chain cysteine residue is in the L-configuration. The amino terminal is acetylated and the carboxyl-terminal is amidated. This compound ("AMG-416") has utility for the treatment of secondary hyperparathyroidism (SHPT) in hemodialysis patients. A liquid formulation comprising AMG-416 may be administered to a subject intravenously. The hydrochloride salt of AMG-416 may be represented as follows:
H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2 · x(HCl)
Therapeutic peptides pose a number of challenges with respect to their formulation. Peptides in general, and particularly those that contain a disulfide bond, typically have only moderate or poor stability in aqueous solution. Peptides are prone to amide bond hydrolysis at both high and low pH.
Disulfide bonds can be unstable even under quite mild conditions (close to neutral pH). In addition, disulfide containing peptides that are not cyclic are particularly prone to dimer formation. Accordingly, therapeutic peptides are often provided in lyophilized form, as a dry powder or cake, for later reconstitution.
A lyophilized formulation of a therapeutic peptide has the advantage of providing stability for long periods of time, but is less convenient to use as it requires the addition of one or more diluents and there is the potential risk for errors due to the use of an improper type or amount of diluent, as well as risk of contamination. In addition, the lyophilization process is time consuming and costly.
H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2
Generic Name:Etelcalcetide
Synonym:KAI-4169
CAS Number:1262780-97-1
N-acetyl-D-cysteinyl-S-(L-cysteine disulfide)-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-D-argininamide
Mechanism of Action:Activates calcium sensing receptor on parathyroid glands reducing PTH synthesis and secretion
Indication: secondary hyperparathyroidism associated with chronic kidney disease
Development Stage: Phase III
Developer:KAI Pharmaceuticals/Amgen Inc.
H-L-Cys-OH
S— S
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2 · x(HCl)

HYDROCHLORIDE
Generic Name:Etelcalcetide Hydrochloride
AMG 416, KAI-4169, previously also known as velcalcetide hydrochloride
CAS :1334237-71-6
Chemical Name:N-acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-D-argininamide disulfide with L-cysteine hydrochloride
Mechanism of Action:Activates calcium sensing receptor on parathyroid glands reducing PTH synthesis and secretion
Indication: secondary hyperparathyroidism associated with chronic kidney disease
Development Stage: Phase III
Developer:KAI Pharmaceuticals/Amgen Inc.
Method for preparing etelcalcetide and its salts, particularly hydrochloride. See WO2014210489, for a prior filing claiming stable liquid formulation of etelcalcetide. Amgen, following its acquisition of KAI Pharmaceuticals, and Japanese licensee Ono Pharmaceuticals are developing etelcalcetide, a long-acting iv isozyme-selective peptide-based protein kinase C epsilon inhibitor and agonist of the calcium-sensing receptor, for treating secondary hyperparathyroidism (SHPT) in patients with end-stage renal disease receiving dialysis.
In August 2015, an NDA was submitted seeking approval of the drug for SHPT in patients with chronic kidney disease (CKD) on hemodialysis (HD) in the US.
In September 2015, Amgen filed an MAA under the centralized procedure in the EU for the approval of etelcalcetide for treating SHPT in patients with CKD on HD therapy.
KAI is also investigating a transdermal patch formulation of the drug for treating primary HPT.
Secondary hyperparathyroidism in patients with chronic kidney disease receiving dialysis
AMG
416 is a peptide agonist of the human cell surface calcium-sensing
receptor (CaSR). It is being investigated as a treatment for secondary
hyperparathyroidism in patients with chronic kidney disease receiving
dialysis.
Etelcalcetide is a novel calcimimetic agent
that suppresses the secretion of parathyroid hormone and is in clinical
development for the treatment of SHPT in patients with CKD on
hemodialysis. Etelcalcetide is administered intravenously three times
per week at the end of each dialysis session. It acts by binding to and
activating the calcium-sensing receptor on the parathyroid gland,
thereby causing decreases in parathyroid hormone (PTH). Sustained
elevations in PTH are known to be associated with significant clinical
consequences for patients with CKD.
The submission includes
data from three Phase 3 studies, all of which met the primary endpoints,
including two pooled placebo-controlled trials in more than 1,000
patients and a head-to-head study evaluating etelcalcetide compared with
cinacalcet.
About Secondary HyperparathyroidismSHPT is
a common and serious condition that is often progressive among patients
with CKD, and it affects many of the approximately two million people
throughout the world who are receiving dialysis, including 450,000
people in the U.S. The disorder develops early in the course of CKD and
usually manifests as increased levels of PTH as a result of increased
production from the parathyroid glands (four small glands in the neck).
Patients with end stage renal disease who require maintenance dialysis
often have substantial elevations of PTH that are commonly associated
with abnormal calcium and phosphorus levels and an increased risk of
significant clinical consequences.
About Etelcalcetide (AMG 416)Etelcalcetide
is a novel calcimimetic agent in clinical development for the treatment
of SHPT in CKD patients on hemodialysis that is administered
intravenously at the end of the dialysis session. Etelcalcetide binds to
and activates the calcium-sensing receptor on the parathyroid gland,
thereby decreasing PTH levels.
About Sensipar® (cinacalcet)Sensipar® (cinacalcet)
is the first oral calcimimetic agent approved by the FDA for the
treatment of SHPT in adult patients with CKD on dialysis. Sensipar is
not indicated for use in adult patients with CKD who are not on dialysis
because of an increased risk of hypocalcemia. The therapy is also
approved in the U.S. for treatment of hypercalcemia in adult patients
with parathyroid carcinoma and hypercalcemia in adult patients with
primary HPT for whom parathyroidectomy would be indicated on the basis
of serum calcium levels, but who are unable to undergo
parathyroidectomy. Sensipar binds to the calcium-sensing receptor,
resulting in a drop in PTH levels by inhibiting PTH synthesis and
secretion. In addition, the reductions in PTH lower serum calcium and
phosphorus levels.
Milestones
- 25 Aug 2015 Preregistration for Secondary hyperparathyroidism in USA (IV)
- 29 May 2015 Pooled analysis efficacy and adverse events data from two phase III trials in secondary hyperparathyroidism released by Amgen
- 21 Apr 2015 Amgen plans to submit Biological License Application to USFDA and Marketing Authorisation Application to EMA for Secondary hyperparathyroidism
PATENT
http://www.google.com/patents/WO2011014707A2?cl=en
PATENT
WO 2015154031https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2015154031&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=PCTDescription
The hydrochloride salt of AMG 416 has the chemical structure:
H-L-Cys-OH
I
s— s
I
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2 · x(HCl)
(SEQ ID NO:l)
The main chain has 7 amino acids, all in the D-configuration. The side-chain cysteine residue is in the L-configuration. The molecular formula of AMG 416 (free base) is C38H73N21O10S2, and has a calculated average molecular mass of 1048.3 Da.
AMG 416 and a method for its preparation are described in International Pat. Publication No. WO 2011/014707, which is incorporated herein by reference for any purpose. As described in International Pat. Publication No. WO 2011/014707, AMG 416 may be assembled by solid-phase synthesis from the corresponding Fmoc-protected D-amino acids. After cleavage from the resin, the material may be treated with Boc-L-Cys(NPyS)-OH to form the disulfide bond. The Boc group may then be removed with trifluoroacetate (TFA) and the resulting product purified by reverse-phase high pressure liquid chromatography (HPLC) and isolated as the TFA salt form by lyophilization. The TFA salt can be converted to a pharmaceutically acceptable salt by carrying out a subsequent salt exchange procedure. Such procedures are well known in the art and include, e.g., an ion exchange technique, optionally followed by purification of the resultant product (for example by reverse phase liquid chromatography or reverse osmosis).
There is a need for an efficient method of producing AMG 416, or a pharmaceutically acceptable salt thereof (e.g., AMG 416 HC1), and particularly one appropriate for commercial scale manufacturing.
In a first aspect, provided is a method for preparing AMG 416, the method comprising: providing a resin-bound peptide having a structure selected from the group consisting of Fmoc-D-Cys(Trt)-D-Ala-D- Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-[Resin] (SEQ ID NO:2) and Ac-D-Cys(Trt)-D-Ala-D- Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-[Resin] (SEQ ID NO:3); cleaving the peptide from the solid support; and activating the side chain of the D-Cys residue of the cleaved peptide.
In a second aspect, provided is a method for preparing AMG 416, the method comprising: providing a peptide having a structure of Ac-D-Cys(SPy)-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2 (SEQ ID NO:4); and contacting the peptide with L-Cys to produce a conjugated product.
In yet a third aspect provided is a method for preparing AMG 416, the method comprising: providing a resin-bound peptide having a structure selected from the group consisting of Fmoc-D-Cys(Trt)-D-Ala-D-Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-[Resin] (SEQ ID NO:2) and Ac-D-Cys(Trt)-D-Ala-D-Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-[Resin] (SEQ ID NO:3); cleaving the peptide from the solid support, i.e., to provide an unsupported peptide, and activating the side chain of the D-Cys residue of the unsupported peptide to generate an AMG 416 SPy intermediate (where SPy is 2-pyridinesulfenyl or S-Pyr), dissolving the AMG 416 SPy intermediate in an aqueous 0.1% TFA (trifluoroacetic acid solution), and purifying the AMG 416 SPy derivative by HPLC.
The term "AMG 416", also known as etelcalcetide, formerly known as velcalcetide or KAI-4169, refers to a compound having the chemical name: N-acetyl-D-cysteinyl-D-alanyl-D-arginyl-D-arginyl-D-arginyl-D-alanyl-D-arginamide disulfide with L-cysteine, which has the following structural formula:
H-L-Cys-OH
I
s— s
I
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2
Reference to AMG 416, or to any compound or AMG 416 fragment, intermediate, or precursor as described herein, is intended to encompass neutral, uncharged forms thereof, as well as pharmaceutically acceptable salts, hydrates and solvates thereof.
The terms "AMG 416 hydrochloride" and "AMG 416 HC1" are interchangeable and refer to a hydrochloride salt form of AMG 416 having the following structural formula:
H-L-Cys-OH
I
s— s
I
Ac-D-Cys-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2 · xHCl
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 shows the chemical structure of AMG 416 (Ac-D-Cys(L-Cys-OH)-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2) (SEQ ID NO: l).

FIG. 2 shows the chemical structure of Rink Amide AM resin and Ac-D-Cys(Trt)- D-Ala-D-Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-Resin (SEQ ID NO:3).
FIG. 3 shows a reaction scheme in which the SPy intermediate product (Ac-D-Cys(SPy)-D-Ala-D-Arg-D-Arg-D-Arg-D-Ala-D-Arg-NH2) (SEQ ID NO:4) is formed from the peptidyl-resin (Ac-D-Cys(Trt)-D-Ala-D-Arg(Pbf)-D-Arg(Pbf)-D-Arg(Pbf)-D-Ala-D-Arg(Pbf)-NH-Resin) (SEQ ID NO:3).
FIG. 4 shows a reaction scheme in which a TFA salt of AMG 416 is formed from the SPy intermediate (AA1_7(SPy)).
FIG. 5 shows a reaction scheme in which the HC1 salt of AMG 416 is formed from the TFA salt of AMG 416.
FIG. 6 shows a reaction scheme in which Boc-D-Arg(Pbf)-OH is formed from Boc-D-Arg-OH.
FIG. 7 shows a reaction scheme in which D-Arg(Pbf)-OH is formed from Boc-D-Arg(Pbf)-OH.
EXAMPLE 5
Purification of the SPy Intermediate and Production of AMG 416 HC1
An alternative method for preparation of AMG 416 HC1 salt is described here. As described in Example 2 above, the SPy intermediate product was dried at 20°C under full vacuum after cleavage from the resin, precipitation and filtration. The precipitate was then dissolved in a 0.1% TFA aqueous solution and loaded onto a C-18 column for HPLC purification. The column was run at <60 bar and the solution temperature was 15-25 °C throughout. The eluents were 0.1% TFA in acetonitrile and 0.1% TFA in water. The fractions were stored at 5°C, they were sampled and then fractions were pooled. The combined pools from two runs were diluted and a concentration/purification run was performed using the same HPLC column to decrease the total volume and remove additional impurities. The fractions were stored at 5°C.
The fractions containing the AMG 416 SPy intermediate were subjected to azeotropic distillation to change the solvent from the 0.1% TFA to a 15% water in IPA solution, charging with IPA as needed. To the resultant AMG 416 SPy intermediate in IPA solution was then added L-Cysteine 1.15 eq and the reaction was allowed to proceed at room temperature for conjugation to occur and to form the AMG 416 TFA salt as described above in Example 4. The AMG 416 TFA solution was added to a solution of 12M aqueous HC1, 0.27 L/kg and IPA 49.4 L/kg over 3 hours via subsurface addition, resulting in direct precipitation of the AMG 416 4.5 HC1 salt. The batch was aged for 3 hours and sampled for analysis.
The material was filtered and slurry washed with 96 wt% IPA, 10 L/kg. The cake was then re-slurried for 4 hours in 10 L/kg of 96% wt% IPA. The material was filtered and further slurry washed with 96% IPA, 10 L/kg and then IPA 10 L/kg. The material was dried under full vacuum at 25°C. The dry cake was dissolved in water 8 L/kg and the batch was concentrated via distillation to remove residual IPA and achieve the desired concentration. The solution temperature was kept below 25 °C throughout the distillation.
PATENT
WO2014210489SEE
https://patentscope.wipo.int/search/en/detail.jsf;jsessionid=2A32CFD9CE075079399E9DD298899C9D.wapp2nC?docId=WO2014210489&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=PCTDescription
EXAMPLE 1
Solubility of AMG 416 in Succinate Buffered Saline
In this study, the solubility of AMG 416 in succinate buffered-saline was investigated. AMG 416 HC1 (103 mg powder, 80 mg peptide) was dissolved in 200 iL of sodium succinate buffered saline (25 mM succinate, 0.9% saline, pH 4.5). After briefly vortexing, a clear solution was obtained with a nominal concentration of 400 mg/mL. Because expansion of the solution volume was not determined, the solubility of AMG 416 can be conservatively stated as at least 200 mg/mL. Although the maximal solubility was not determined in this experiment, AMG 416 is soluble in pH 4.5 succinate buffered saline to concentrations of at least 200 mg/mL.
REFERENCES
- "Amgen Submits New Drug Application For Novel Intravenous Calcimimetic Etelcalcetide (AMG 416)”
- "Velcalcetide (AMG 416), a novel peptide agonist of the calcium-sensing receptor, reduces serum parathyroid hormone and FGF23 levels in healthy male subjects
- "Evidence for Chronic Kidney Disease-Mineral and Bone Disorder Associated With Metabolic Pathway Changes”
49th Congr Eur Renal Assoc - Eur Dialysis Transpl Assoc (May 24-27, Paris) 2012, Abst SAO054
KAI-4169,
a novel peptide agonist of the calcium sensing receptor, attenuates PTH
and soft tissue calcification and restores parathyroid gland VDR levels
in uremic rats
49th Congr Eur Renal Assoc - Eur Dialysis Transpl Assoc (May 24-27, Paris) 2012, Abst SAO014
49th Congr Eur Renal Assoc - Eur Dialysis Transpl Assoc (May 24-27, Paris) 2012, Abst SAO014
Long
term safety and efficacy of velcalcetide (AMG 416), a calcium-sensing
receptor (CaSR) agonist, for the treatment of secondary
hyperparathyroidism (SHPT) in hemodialysis (HD) patients
Kidney Week (November 5-10, Atlanta, GA) 2013, Abst SA-PO575
Kidney Week (November 5-10, Atlanta, GA) 2013, Abst SA-PO575
Preclinical PK and PD relationship for KAI-4169, a novel calcimimetic
93rd Annu Meet Endo Soc (June 4-7, Boston) 2011, Abst P1-198
93rd Annu Meet Endo Soc (June 4-7, Boston) 2011, Abst P1-198
KAI-4169, a novel calcimimetic for the treatment of secondary hyperparathyroidism
93rd Annu Meet Endo Soc (June 4-7, Boston) 2011, Abst P2-98
93rd Annu Meet Endo Soc (June 4-7, Boston) 2011, Abst P2-98
Characterization
of KAI-4169, a novel peptide for the treatment of chronic kidney
disease - Mineral and bone disorder, in a phase I study in healthy males
44th Annu Meet Am Soc Nephrol (ASN) (November 8-13, Philadelphia) 2011, Abst FR-PO1238
44th Annu Meet Am Soc Nephrol (ASN) (November 8-13, Philadelphia) 2011, Abst FR-PO1238
| WO2011014707A2 | Jul 29, 2010 | Feb 3, 2011 | Kai Pharmaceuticals, Inc. | Therapeutic agents for reducing parathyroid hormone levels |
//////////////Etelcalcetide, AMG 416, KAI-4169, velcalcetide
Inhibitor peptide corresponding to last 10 amino acids of the C-terminus of the GluA2 AMPA receptor subunit that inhititss binding of GluA2 (at the C-terminal PDZ site) to glutamate receptor interacting protein (GRIP), AMPA receptor binding protein (ABP) and protein interacting with C kinase (PICK1). pep2-SVKI
ReplyDelete