
(1R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine
Eisai Expands Marketing and Supply Agreement for Anti-obesity Agent Lorcaserin to Include Most Countries Worldwide
HATFIELD, England, November 8, 2013 /PRNewswire/ --
Eisai announces today that it has expanded the marketing and supply agreement between its U.S. subsidiary Eisai Inc. and U.S-based Arena Pharmaceuticals Inc.'s Swiss subsidiary, Arena Pharmaceuticals GmbH, for the anti-obesity agent lorcaserin hydrochloride (lorcaserin) (U.S. brand name: BELVIQ®). Whilst the existing agreement granted Eisai Inc. exclusive rights to market and distribute lorcaserin in 21 countries throughout the Americas, the expanded agreement now includes most countries and territories worldwide, most notably, the member states of the European Union, Japan and China (but excludes South Korea, Taiwan, Australia, New Zealand and Israel).http://www.pharmalive.com/eisai-expands-lorcaserin-marketing-and-supply-agreement
Lorcaserin (previously APD-356), a highly selective 5HT2C receptor agonist, is used for the treatment of obesity. It has been shown to reduce body weight and food intake in animal models of obesity, and it is thought that targeting the 5HT2C receptor may alter body weight by regulating satiety. Lorcaserin is marketed as a salt form called Belviq, which is lorcaserin hydrochloride.
Lorcaserin (APD-356, trade name upon approval Belviq, expected trade name during development, Lorqess) is aweight-loss drug developed by Arena Pharmaceuticals. It has serotonergic properties and acts as an anorectic. On 22 December 2009 a New Drug Application (NDA) was submitted to the Food and Drug Administration (FDA) in the United States. On 16 September 2010, an FDA advisory panel voted to recommend against approval of the drug based on concerns over both safety and efficacy. In October 2010, the FDA stated that it could not approve the application for lorcaserin in its present form.anti-obesity drug that Arena Pharmaceuticals is creation, Eisai Co., Ltd. has the right to sell "BELVIQ ®" (generic name lorcaserin hydrochloride) was to get the FDA approval on June 27, 2012
On 10 May 2012, after a new round of studies submitted by Arena, an FDA panel voted to recommend lorcaserin with certain restrictions and patient monitoring. The restrictions include patients with a BMI of over 30, or with a BMI over 27 and a comorbidity like high blood pressure or type 2 diabetes.
On 27 June 2012, the FDA officially approved lorcaserin for use in the treatment of obesity for adults with a BMI equal to or greater than 30 or adults with a BMI of 27 or greater who "have at least one weight-related health condition, such as high blood pressure, type 2 diabetes, or high cholesterol".
On 7 May 2013, the US Drug Enforcement Administration has classified lorcaserin as a Schedule IV drug under the Controlled Substances Act.
Obesity is a life-threatening disorder in which there is an increased risk of morbidity and mortality arising from concomitant diseases such as type II diabetes, hypertension, stroke, cancer and gallbladder disease.
Obesity is now a major healthcare issue in the Western World and increasingly in some third world countries. The increase in numbers of obese people is due largely to the increasing preference for high fat content foods but also the decrease in activity in most people's lives. Currently about 30% of the population of the USA is now considered obese.
Whether someone is classified as overweight or obese is generally determined on the basis of their body mass index (BMI) which is calculated by dividing body weight (kg) by height squared (m2). Thus, the units of BMI are kg/m2 and it is possible to calculate the BMI range associated with minimum mortality in each decade of life. Overweight is defined as a BMI in the range 25-30 kg/m2, and obesity as a BMI greater than 30 kg/m2 (see table below).
Classification Of Weight By Body Mass Index (BMI)

As the BMI increases there is an increased risk of death from a variety of causes that are independent of other risk factors. The most common diseases associated with obesity are cardiovascular disease (particularly hypertension), diabetes (obesity aggravates the development of diabetes), gall bladder disease (particularly cancer) and diseases of reproduction. The strength of the link between obesity and specific conditions varies. One of the strongest is the link with type 2 diabetes. Excess body fat underlies 64% of cases of diabetes in men and 77% of cases in women (Seidell, Semin Vase Med, 5:3-14 (2005)). Research has shown that even a modest reduction in body weight can correspond to a significant reduction in the risk of developing coronary heart disease.
Various synthetic routes to (R)-8-chloro-l -methyl-2,3,4,5-tetrahydro-lH-3-benzazepine, its related salts, enantiomers, crystalline forms, and intermediates, have been reported in WO 2005/019179 WO2003/086303, WO 2006/069363, WO 2007/120517, WO 2008/07011 1 , WO 2009/111004, and WO 2010/148207 each of which is incorporated herein by reference in its entirety. Combinations of (R)-8-Chloro-l -methyl-2,3,4,5-tetrahydro-lH-3-benzazepine with other agents, including without limitation, phentermine, and uses of such combinations in therapy are described in WO 2006/071740, which is incorporated herein by reference in its entirety.


BELVIQ (lorcaserin hydrochloride) is a serotonin 2C receptor agonist for oral administration used for chronic weight management. Its chemical name is (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride hemihydrate. The empirical formula is C11H15Cl2N•0.5H2O, and the molecular weight of the hemihydrate form is 241.16 g/mol.
The structural formula is:
 |
METHOD FOR CHRONIC WEIGHT MANAGEMENT BY DECREASING FOOD INTAKE U1252
| Patent No US | PatentExpiry Date | patent use code |
|---|---|---|
| 7514422 | Apr 10, 2023 | U-1252 |
| 7977329 | Apr 10, 2023 | U-1252 |
| 8168624 | Apr 18, 2029 | |
| 8207158 | Apr 10, 2023 | U-1252 |
| 8273734 | Apr 10, 2023 | U-1254 |
| Exclusivity Code | Exclusivity_Date |
|---|---|
| NCE | Jun 27, 2017 |
1
Various synthetic routes to (R)-8-chloro-l-methyl-2,3,4,5-tetrahydro-lH-3-benzazepine, its related salts, enantiomers, crystalline forms, and intermediates, have been reported in PCT publications, WO 2005/019179, WO 2006/069363, WO 2007/120517, WO 2008/070111 , WO 2009/111004, and in United States provisional application 61/396,752 each of which is incorporated herein by reference in its entirety.
Combinations of (R)-8-Chloro-l -methyl-2,3,4,5-tetrahydro-lH-3-benzazepine with other agents, including without limitation, phentermine, and uses of such combinations in therapy are described in WO 2006/071740, which is incorporated herein by reference in its entirety
The following United States provisional applications are related to (R)-8-chloro-l- methyl-2,3,4,5-tetrahydro-lH-3-benzazepine: 61/402,578; 61/403,143; 61/402,580; 61/402,628; 61/403,149; 61/402,589; 61/402,611 ; 61/402,565; 61/403, 185; each of which is incorporated herein by reference in its entirety.
| Date | Supplement No. | Action | Documents |
|---|---|---|---|
| 2012-06-27 | 000 | Approval | |
| 2013-01-04 | 001 | Manufacturing Change or Addition | |
| 2013-11-01 | 002 | Manufacturing Change or Addition |
Various synthetic routes to (R)-8-chloro-l -methyl-2,3,4,5-tetrahydro-lH-3-benzazepine, its related salts, enantiomers, crystalline forms, and intermediates, have been reported in WO 2005/019179, WO 2006/069363, WO 2007/120517, WO 2008/07011 1 , WO 2009/111004, and WO 2010/148207 each of which is incorporated herein by reference in its entirety. Combinations of (R)-8-Chloro-l -methyl-2,3,4,5-tetrahydro-lH-3-benzazepine with other agents, including without limitation, phentermine, and uses of such combinations in therapy are described in WO 2006/071740, which is incorporated herein by reference in its entirety.
3-Benzazepines have been found to be agonists of the 5HT2C receptor and show effectiveness at reducing obesity in animal models (see, e.g., U.S. Ser. No. 60/479,280 and U.S. Ser. No. 10/410,991, each of which is incorporated herein by reference in its entirety). Numerous synthetic routes to 3-benzazepines have been reported and typically involve a phenyl-containing starting material upon which is built an amine- or amide-containing chain that is capable of cyclizing to form the fused 7-member ring of the benzazepine core. Syntheses of 3-benzazepines and intermediates thereof are reported in U.S. Ser. No. 60/479,280 and U.S. Ser. No. 10/410,991 as well as Nair et al., Indian J. Chem., 1967, 5, 169; Orito et al., Tetrahedron, 1980, 36, 1017; Wu et al., Organic Process Research and Development,1997, 1, 359; Draper et al., Organic Process Research and Development, 1998, 2, 175; Draper et al., Organic Process Research and Development, 1998, 2, 186; Kuenburg et al., Organic Process Research and Development, 1999, 3, 425; Baindur et al., J. Med. Chem.,1992, 35(1), 67; Neumeyer et al., J. Med. Chem., 1990, 33, 521; Clark et al., J. Med. Chem.,1990, 33, 633; Pfeiffer et al., J. Med. Chem., 1982, 25, 352; Weinstock et al., J. Med. Chem., 1980, 23(9), 973; Weinstock et al., J. Med. Chem., 1980, 23(9), 975; Chumpradit et al., J. Med. Chem., 1989, 32, 1431; Heys et al., J. Org. Chem., 1989, 54, 4702; Bremner et al., Progress in Heterocyclic Chemistry, 2001, 13, 340; Hasan et al., Indian J. Chem., 1971, 9(9), 1022; Nagle et al., Tetrahedron Letters, 2000, 41, 3011; Robert, et al., J. Org. Chem., 1987, 52, 5594); and Deady et al., J. Chem. Soc., Perkin Trans. I, 1973, 782.
Other routes to 3-benzazepines and related compounds are reported in Ladd et al., J. Med. Chem., 1986, 29, 1904; EP 204349; EP 285 919; CH 500194; Tetrahedron Letters, 1986, 27, 2023; Ger. Offen., 3418270, 21 Nov. 1985; J. Org. Chem.,1985, 50, 743; U.S. Pat. Nos. 4,957,914 and 5,015,639; Synthetic Commun., 1988, 18, 671; Tetrahedron, 1985, 41, 2557;Hokkaido Daigaku Kogakubu Kenhyu Hokoku, 1979, 96, 414; Chemical & Pharmaceutical Bulletin, 1975, 23, 2584; J. Am. Chem. Soc., 1970, 92, 5686; J. Am. Chem. Soc., 1968, 90, 6522; J Am. Chem. Soc., 1968, 90, 776; J. Am. Chem. Soc.,1967, 89, 1039; and Chang et al., Bioorg. Med. Chem. Letters, 1992, 2, 399

Its synthesis starting from compound 1 and, via SN2 coupling to form 3, thionyl chloride, to form 4, aluminum chloride catalyzed Friedel-Crafts alkylation ring closure to give the racemic product 5, through L- tartaric acid separation, obtained chiral
SYNTHESIS
Smith, J.; Smith, B. 5HT2C receptor modulators. U.S. Patent 2003225057, 2003.
Smith, B.; Smith, J. 5HT2C receptor modulators. U.S. Patent 6953787, 2005
PATENT
http://www.google.com/patents/US8367657Example 6 Preparation of 2-(4-Chlorophenyl)-N-ethyl-N-2-propylchloride
To a dry 100-milliliter, round bottom flask under nitrogen with stirring was added 2-(4-chlorophenyl)ethyl-N-2-chloropropionylamide (8.8 g, 35.8 mmol) followed by borane in THF (1.8 M, 70 mL, 140 mmol) over 10 minutes (gas evolution and solid becomes solubilized). After the addition was complete, boron trifluoride in tert-butyl methyl ether (8 mL, 70.8 mmol) was added over 10 minutes with more gas evolution. After 4 hours, LC/MS showed complete reaction. The reaction mixture was quenched with 20 mL of conc. HCL (37%) with additional of gas evolution. The reaction mixture was stirred at 40° C. for 2 hours, cooled to room temperature and evaporated. Then, the white slurry was taken up in 40 mL ethyl acetate and 20 mL of 2.5 M NaOH to make a yellow solution over a white slurry. The yellow organic layer was washed with brine, dried over magnesium sulfate, filtered and evaporated to give 12.2 grams of white to yellow solid. This solid was recrystallized from ethyl acetate/hexane in two crops to give 6.7 grams of white solid product (80% yield).
1H NMR (DMSO-d6): 9.0 (br s, 2 H, NH, HCl), 7.2 (d, 2H, J=8 Hz), 7.05 (d, 2H, J=8 Hz), 4.5 (m, 1H), 3.2 (m, 2H), 3.1 (m, 2H), 3.0 (m, 2H), 1.5 (d, 3H, J=7 Hz).
LC/MS: 1.71 minute, 232.1 M+H+ and 139 major fragment. Minor impurity observed at 2.46 min with 321 and 139 peaks.
Example 1 Preparation of 2-(4-chlorophenyl)ethyl-N-2-chloropropionamide
To a 1-liter, 3-necked round bottom flask under argon balloon equipped with reflux condenser and addition funnel, were added sequentially 2-(4-chlorophenyl) ethylamine (30 g, 193 mmol), 400 mL acetonitrile, triethylamine (19.5 g, 193 mmol) and 80 mL acetonitrile. The clear colorless solution was stirred and cooled to 0° C. 2-Chloropropionyl chloride (24.5 g, 193 mmol, distilled) in 5 mL acetonitrile was slowly added over 20 minutes to evolution of white gas, formation of white precipitate, and color change of reaction mixture to slight yellow. An additional 10 mL of acetonitrile was used to rinse the addition funnel. The mixture was stirred at 0° C. for 30 minutes and then warmed to room temperature and stirred vigorously for an additional one hour. The yellow reaction mixture was concentrated on the rotary evaporator to a solid containing triethylamine hydrochloride (76.36 grams). This material was taken up in 100 mL ethylacetate and 200 mL water, and stirred vigorously. The layers were separated and the aqueous layer was extracted with an additional 100 mL ethylacetate. The combined organic layers were washed twice with 25 mL of saturated brine, dried over magnesium sulfate, filtered, and concentrated to a light tan solid (41.6 grams, 88%). TLC in ethylacetate-hexane, 8:2 showed a major spot two-thirds of the way up the plate and a small spot at the baseline. Baseline spot was removed as follows: This material was taken up in 40 mL of ethylacetate and hexane was added until the solution became cloudy. Cooling to 0° C. produced a white crystalline solid (40.2 grams, 85% yield). The product is a known compound (Hasan et al., Indian J. Chem., 1971, 9(9), 1022) with CAS Registry No. 34164-14-2.
LC/MS gave product 2.45 minute; 246.1 M++H+.
1H NMR (CDCl3): δ 7.2 (dd, 4H, Ar), 6.7 (br S, 1H, NM, 4.38 (q, 1H, CHCH3), 3.5 (q, 2H, ArCH2CH2NH), 2.8 (t, 2H, ArCH2), 1.7 (d, 3H, CH3).
13C NMR (CDCl3): 169 (1C, C═O), 136 (1C, Ar—Cl), 132 (1C, Ar), 130 (2C, Ar), 128 (2C, Ar), 56 (1C, CHCl), 40 (1C, CHN), 34 (1C, CHAr), 22 (1C, CH3).
Example 2 Preparation of 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepin-2-one
2-(4-Chlorophenyl)ethyl-N-2-chloropropionamide (10 g, 40.6 mmol) of Example 1 and aluminum chloride (16 g, 119.9 mmol) were added to a clean dry 100 mL round bottom flask equipped with an argon balloon, stirring apparatus, and heating apparatus. The white solid melted to a tan oil with bubbling at 91° C. (Note: if impure starting materials are used, a black tar can result but clean product can still be isolated). The mixture was heated and stirred at 150° C. for 12 hours. (Note: The time is dependent on the reaction scale and can easily be followed by LC/MS; higher temperatures can be used for shorter time periods. E.g., a 1 gram sample was complete in 5 hours.) The reaction can be followed by LC/MS with the starting material at 2.45 minutes (246.1 M++H+), the product at 2.24 minutes (209.6 M++H+) on a 5 minute reaction time from 5-95% w/0.01% TFA in water/MeCN (50:50).
After cooling to room temperature, the reaction mixture was quenched with slow addition of 10 mL of MeOH followed by 5 mL of 5% HCl in water and 5 mL of ethyl acetate. After separation of the resulting layers, the aqueous layer was extracted a second time with 10 mL of ethyl acetate. The combined organic layers were dried over magnesium sulfate, filtered, and concentrated to a tan solid (6.78 grams, 80% yield). LC/MS showed one peak, at 2.2 min and 209.6 MI. This material was taken up in ethyl acetate, filtered through celite and Kieselgel 60 (0.5 inch plug on a 60 mL Buchner funnel) and the filtrate was recrystallized from hexane/ethyl acetate to give final product (4.61 grams, 54% yield).
1H NMR (CDCl3): 7.3-7.1 (m, 3H, Ar), 5.6 (br S, 1H, NH), 4.23 (q, 1H, CHCH3), 3.8 (m, 1H, ArCH2CH 2NH), 3.49 (m, 1H, ArCH2CH 2NH), 3.48 (m, 1H, ArCH 2CH2NH), 3.05 (m, 1H, ArCH 2CH2NH), 1.6 (d, 3H, CH2).
13C NMR (CDCl3): 178 (1C, C═O), 139 (1C, Ar), 135 (1C, Ar), 130, 129 (2C, Ar), 126 (2C, Ar), 42 (1C, C), 40 (1C, CHN), 33 (1C, CHAr), 14 (1C, CH3).
Example 3 Preparation of 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine
Procedure A
HPLC purified 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazapin-2-one (150 mg, 0.716 mmol) of Example 2 was added to a 50 mL round bottom flask with 2M borane-tetrahydrofuran solution (2 mL, 2.15 mmol). The mixture was stirred 10 hours at room temperature under an argon balloon. LC/MS showed the desired product as the major peak with approximately 5% of starting material still present. The reaction mixture was quenched with 5 mL methanol and the solvents were removed on the rotary evaporator. This procedure was repeated with methanol addition and evaporation. The mixture was evaporated on the rotary evaporator followed by 2 hours in vacuo to give the product as a white solid (117 mg, 70% yield).
NMR, LC/MS and other analytical data are provided below.
Procedure B
Recrystallized 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazapin-2-one (137 mg, 0.653 mmol) was added to a 50 mL round bottom flask with stirring under nitrogen gas. To the flask was slowly added borane-tetrahydrofuran solution (1M, 10 mL) followed by boron trifluoride TBME solution (1 mL, 8.85 mmol) with vigorous gas evolution. The mixture was stirred 6 hours at room temperature under nitrogen gas. LC/MS showed the desired product. The reaction mixture was quenched with 5 mL methanol and 3 mL conc. HCl and the solvents were removed on the rotary evaporator. This procedure was repeated with methanol and HCl addition and evaporation. The mixture was evaporated on the rotary evaporator followed by 2 hours on the pump to dryness to give 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazapine hydrochloride (106 mg, 70% yield).
1H NMR (CDCl3): 10.2 (br s, 1H), 9.8 (br s, 1H), 7.14 (dd, 1H, J=2, 8 Hz), 7.11 (d, 1H, J=2 Hz), 7.03 (d, 1H, J=8 Hz), 3.6 (m, 2H), 3.5 (m, 2H), 2.8-3.0 (m, 3 H), 1.5 (d, 3H, J=7 Hz).
LC/MS: 1.41 minute, 196.1 M+H+and 139 major fragment. No impurities were observed.
Example 4 Preparation of L-(+)-tartaric acid salt of (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine
To a clean, dry 50 mL round bottom flask were added 11.5 g (0.06 mol) of 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine from Example 3 to 2.23 g (0.015 mol) of L-(+)-tartaric acid. The suspension was diluted with 56 g of tert-butanol and 6.5 mL of H2O. The mixture was heated to reflux (75-78° C.) and stirred for 10 min to obtain a colorless solution. The solution was slowly cooled down to room temperature (during 1 h) and stirred for 3 h at room temperature. The suspension was filtered and the residue was washed twice with acetone (10 mL). The product was dried under reduced pressure (50 mbar) at 60° C. to yield 6.3 g of the tartrate salt (ee=80). This tartrate salt was added to 56 g of tert-butanol and 6.5 mL of H2O. The resulting suspension was heated to reflux and 1 to 2 g of H2O was added to obtain a colorless solution. The solution was slowly cooled down to room temperature (over the course of 1 h) and stirred for 3 h at room temperature. The suspension was filtered and the residue was washed twice with acetone (10 mL). The product was dried under reduced pressure (50 mbar) at 60° C. to produce 4.9 g (48% yield) of product (ee>98.9).
If the ee value of the product obtained is not satisfactory, an additional recrystallization can be carried out as described. Either enantiomer can be synthesized in high ee utilizing this method.
Example 5 Conversion of Salt Form to Free Amine
The L-tartaric acid salt of 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine (300 mg, 0.87 mmol) from Example 4 was added to a 25 mL round bottom flask with 50% sodium hydroxide solution (114 μL, 2.17 mmol) with an added 2 mL of water. The mixture was stirred 3 minutes at room temperature. The solution was extracted with methylene chloride (5 mL) twice. The combined organic extracts were washed with water (5 mL) and evaporated to dryness on the pump to get free amine (220 mg crude weight). LC/MS 196 (M+H).
Example 14 Preparation of Hydrochloric Acid Salt of (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine
To a clean, dry 25 mL round bottom flask were added (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine free amine (220 mg), 3 ML methylene chloride, and 1.74 mL of 1M HCl in ether. The mixture was stirred for 5 minutes at room temperature. The solvent was removed under reduced pressure to give a white solid, the HCl salt. The salt was re-dissolved in methylene chloride (3 mL) and an additional 1.74 mL of 1 M HCl was added and the solution was again stirred at room temperature for 5 minutes. The solvent was removed under reduced pressure to give the desired HCl salt of 8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazapine (190 mg crude weight, 95% yield). NMR data was consistent with the desired product.
1H NMR (CDCl3): 10.2 (br s, 1H), 9.8 (br s, 1H), 7.14 (dd, 1H, J=2, 8 Hz), 7.11 (d, 1H, J=2 Hz), 7.03 (d, 1H, J=8 Hz), 3.6 (m, 2H), 3.5 (m, 2H, 2.8-3.0 (m, 3 H), 1.5 (d, 3H, J=7 Hz).
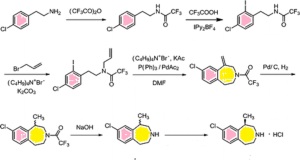
Paper
A novel synthesis of antiobesity drug lorcaserin hydrochloride was accomplished in six steps.N-protection of 2-(4-chlorophenyl)ethanamine with di-tert-butyl dicarbonate, N-alkylation with allyl bromide, deprotection, intramolecular Friedel–Crafts alkylation, chiral resolution with l-(+)-tartaric
acid, and the final salification led to the target molecule lorcaserin
hydrochloride in 23.1% overall yield with 99.9% purity and excellent
enantioselectivity (>99.8% ee). This convenient and economical
procedure is remarkably applicable for scale-up production.
Org. Process Res. Dev., 2015, 19 (9), pp 1263–1267
DOI: 10.1021/acs.oprd.5b00144
Lorcaserin hydrochloride
(R)-8-Chloro-1-methyl-2,3,4,5-tetrahydro-1H-benzo[d]azepinehydrochloride (1)
To a solution of 16 (0.66 kg, 2.44 mol) in water (3 L) was added 20% K2CO3
aqueous solution. The pH was adjusted to 8–9 and extracted with
cyclohexane (5 L × 2). The combined organic layer was washed with brine
(5 L × 2), dried with anhydrous Na2SO4, filtered,
and concentrated to afford lorcaserin as yellow oil. To a stirred
solution of lorcaserin free base in anhydrous ethanol (500 mL) was added
HCl-saturated EtOAc solution slowly until pH = 2 and stirred for
another 5 h at room temperature. The reaction solution was concentrated
and then stirred for 1 h in methyl tert-butyl ether (2 L) at room temperature. The precipitate was filtered, washed with methyl tert-butyl ether (200 mL), and dried under vacuum to give lorcaserin hydrochloride (1) (0.52 kg, 91.2%). HPLC purity: 99.9%, chiral purity: 99.9%. Mp: 198–199 °C.
1H NMR (300 MHz, DMSO-d6): δ = 9.61 (bs, 2H), 7.28–7.21 (m, 3H), 3.54–3.44 (m, 1H), 3.33–3.18 (m, 3H), 3.01 (dd, J = 15.7, 7.1 Hz, 1H), 2.91–2.83 (m, 2H), 1.34 (d, J = 7.2 Hz, 3H).
13C NMR (75 MHz, DMSO-d6): δ = 145.4, 138.1, 131.5, 126.4, 126.0, 114.5, 50.1, 44.5, 34.1, 30.8, 17.5.
MS (ESI, 70 eV): m/z = 196.1 [M + H]+.
HPLC for 1 (tR
= 9.0 min) purity 99.9%: Intersil ODS-3 5 μm C-18 250 mm × 4.6 mm, flow
rate = 1 mL/min, 35 °C, gradient elution from 20:88 A/B for 30 min to
75:25 A/B over 30 min; A = acetonitrile; B = phosphoric acid in water
(pH = 6.0); UV λ = 220 nm.
Chiral HPLC for 1 (tR = 21.6 min) purity 99.9%: Daicel AD-RH 5 μm 250 mm × 4.6 mm, flow rate = 1 mL/min, 35 °C, isocratic A/B/C = 92:8:0.1; A = n-hexane; B = isopropanol; C = diethylamine; UV λ = 220 nm.
| WO2010148207A2 | 17 Jun 2010 | 23 Dec 2010 | Arena Pharmaceuticals, Inc. | Processes for the preparation of 5-ht2c receptor agonists |
| WO2011153206A1 | 1 Jun 2011 | 8 Dec 2011 | Arena Pharmaceuticals, Inc. | Processes for the preparation of 5-ht2c receptor agonists |
| WO2012030927A2 | 31 Aug 2011 | 8 Mar 2012 | Arena Pharmaceuticals, Inc. | Modified-release dosage forms of 5-ht2c agonists useful for weight management |
| WO2012030938A1 | 31 Aug 2011 | 8 Mar 2012 | Arena Pharmaceuticals, Inc. | Salts of lorcaserin with optically active acids |
| WO2012030939A1 | 31 Aug 2011 | 8 Mar 2012 | Arena Pharmaceuticals, Inc. | Administration of lorcaserin to individuals with renal impairment |
| WO2012030951A1 | 31 Aug 2011 | 8 Mar 2012 | Arena Pharmaceuticals, Inc. | Fast-dissolve dosage forms of 5-ht2c agonists |
| WO2012030953A1 | 31 Aug 2011 | 8 Mar 2012 | Arena Pharmaceuticals, Inc. | 5-ht2c receptor agonists in the treatment of disorders ameliorated by reduction of norepinephrine level |
| WO2012030957A2 | 31 Aug 2011 | 8 Mar 2012 | Arena Pharmaceuticals, Inc. | Non-hygroscopic salts of 5-ht2c agonists |
| EP2443080A2 * | 17 Jun 2010 | 25 Apr 2012 | Arena Pharmaceuticals, Inc. | Process for the preparation of 5-ht2c receptor agonists |
| WO2007120517A2 * | 2 Apr 2007 | 25 Oct 2007 | Arena Pharm Inc | Processes for the preparation of 8-chloro-1-methyl-2,3,4,5-tetrahydro-1h-3-benzazepine and intermediates related thereto |

back to home for more updates

DR ANTHONY MELVIN CRASTO Ph.D
amcrasto@gmail.com
MOBILE-+91 9323115463
GLENMARK SCIENTIST , NAVIMUMBAI, INDIA








No comments:
Post a Comment