
Ravidasvir dihydrochloride
C42H50N8O6.2(HCl), 835.83
CAS 1303533-81-4
Phase II/IIIHepatitis C

Ravidasvir
PPI-668 free base; BI 238630;
CAS:1242087-93-9
PPI-668 free base; BI 238630;
CAS:1242087-93-9
C42H50N8O6, 762.38
Chemical Name:methyl N-[(1S)-1-({(2S)-2-[5-(6-{2-[(2S)-1-{(2S)-2-[(methoxycarbonyl)amino]- 3- methylbutanoyl}pyrrolidin-2-yl]-1H-imidazol-4-yl}naphthalen-2-yl) -1H- benzimidazol- 2-yl]pyrrolidin-1-yl}carbonyl)-2-methylpropyl]carbamate
Mechanism of Action:NS5A Inhibitor
Indication: hepatitis C
Development Stage: Phase II
Developer:Presidio Pharmaceuticals, Inc
Chemical Name:methyl N-[(1S)-1-({(2S)-2-[5-(6-{2-[(2S)-1-{(2S)-2-[(methoxycarbonyl)amino]- 3- methylbutanoyl}pyrrolidin-2-yl]-1H-imidazol-4-yl}naphthalen-2-yl) -1H- benzimidazol- 2-yl]pyrrolidin-1-yl}carbonyl)-2-methylpropyl]carbamate
Mechanism of Action:NS5A Inhibitor
Indication: hepatitis C
Development Stage: Phase II
Developer:Presidio Pharmaceuticals, Inc
- OriginatorXTL Biopharmaceuticals
- Developer Pharco Corporation; Presidio Pharmaceuticals
- Class Antivirals; Benzimidazoles; Carbamates; Naphthalenes; Pyrrolidines; Small molecules
- Mechanism of Action Hepatitis C virus NS 5 protein inhibitors; Hepatitis C virus replication inhibitors
- 31 Aug 2015 Ascletis plans to initiate the phase II EVEREST trial for Hepatitis C (Combination therapy; Treatment-naive) in Taiwan
- 31 Aug 2015 Taiwan Food and Drug Administration approves Clinical Trial Application to initiate a phase II trial for interferon free regimen comprising danoprevir and ravidasvir in Hepatitis C
- 24 Jun 2015 Efficacy data from a phase IIa trial in Hepatitis C released by Ascletis
Ravidasvir [Methyl N-[(1S)-1-({(2S)-2-[5-(6-{2-[(2S)-1-{(2S)-2-[(methoxycarbonyl)amino]- 3- methylbutanoyl}pyrrolidin-2-yl]-1H-imidazol-4-yl}naphthalen-2-yl) -1H- benzimidazol- 2-yl]pyrrolidin-1-yl}carbonyl)-2-methylpropyl]carbamate] is an Nonstructural protein 5A (NS5A) inhibitor. It is an antiviral agent that is being developed as a potential treatment for hepatitis C virus infection.
PPI-668, a non-structural 5A (NS5A) protein of hepatitis C virus (HCV) inhibitor, is in phase II clinical studies at Presidio Pharmaceuticals for the treatment of chronic genotype 1 hepatitis C virus infection.
Ravidasvir has 50% inhibitory concentrations (EC50s) values of 0.02-1.3 nM in replicon assays for HCV genotypes 1-7 (gt1-gt7).
PPI-668, a non-structural 5A (NS5A) protein of hepatitis C virus (HCV) inhibitor, is in phase II clinical studies at Presidio Pharmaceuticals for the treatment of chronic genotype 1 hepatitis C virus infection.
Ravidasvir has 50% inhibitory concentrations (EC50s) values of 0.02-1.3 nM in replicon assays for HCV genotypes 1-7 (gt1-gt7).
Ravidasvir was developed by Presidio Pharmaceuticals Inc, later Ascletis licensed it. Ravidasvir is in Phase II clinical trials proving interferon (IFN)-free regimen to treat chronic hepatitis C (CHC). Ascletis is now the first Chinese company to file clinical trial applications in China for an IFN-free regimen.
In 2014, Ascletis acquired rights for development and commercialization in Greater China and Pharco in Egypt for the treatment of hepatitis C.
Hepatitis C virus infection is a major health problem worldwide and no vaccine has yet been developed against this virus. The standard therapy of pegylated-interferon and ribavirin induces serious side effects and provides viral eradication in less than 50% of patients. Combination therapy of HCV including ribavirin and interferon are currently is the approved therapy for HCV. Unfortunately, such combination therapy also produces side effects and is often poorly tolerated, resulting in major clinical challenges in a significant proportion of patients. The combination of direct acting agents can also result in drug-drug interactions. To date, no HCV therapy has been approved which is interferon free. There is therefore a need for new combination therapies which have reduced side effects, and interferon free, have a reduced emergence of resistance, reduced treatment periods and/or and enhanced cure rates.
Nonstructural protein 5A (NS5A) is a zinc-binding and proline-rich hydrophilic phosphoprotein that plays a key role in Hepatitis C virus RNA replication.
A number of direct-acting antiviral agents (DAAs) are under development for the treatment of chronic HCV infection. These agents block viral production by directly inhibiting one of several steps of the HCV lifecycle. several viral proteins involved in the HCV lifecycle, such as the non-structural (NS)3/4A serine protease, the NS5B RNA-dependent RNA polymerase (RdRp), and the NS5A protein, have been targeted for drug development. Two NS3/4A protease inhibitors already approved for clinical use, numerous other protease inhibitors are being developed as well as inhibitors of viral replication, including nucleoside/nucleotide analogue inhibitors of HCV RdRp, non-nucleoside inhibitors of RdRp, cyclophilin inhibitors, and NS5A inhibitors.
Inhibition of NS5A at picomolar concentrations has been associated with significant reductions in HCV RNA levels in cell culture-based models, which makes these agents among the most potent antiviral molecules yet developed.
Activity:
This NS5A inhibitor has been shown to possess high efficacy against HCV genotype 1, with up to 3.7 log10 mean HCV RNA reductions, in a Phase Ib clinical trial. Activity was demonstrated against variants harbouring the L31M substitution. In an added genotype-2/3 cohort, the first 2 patients achieved mean 3.0 log10 RNA level reductions [1].
Results from the Phase IIa study involving a combination therapy with Faldaprevir and Deleobuvir plus Ravidasvir came with positive news where the said combination cured 92 percent of those with genotype 1a of hepatitis C virus (HCV) when given with ribavirin. The results presented at the 49th annual meeting of the European Association for the Study of the Liver (EASL) in London [2, 3].
The 36 study participants were randomly dived into three even cohorts of 12 each: The first received 600 mg of Deleobuvir twice a day as well as once-daily doses of Faldaprevir (120 mg), Ravidasvir and Ribavirin. The second group received the same regimen except the Faldaprevir dose was 400 mg. The third group took the regimen with the higher dose of Faldaprevir, but without Ribavirin. All participants were treated for 12 weeks with follow up for next 24 weeks.
Ninety-two percent of the first and second cohorts (11 out of 12 in both cases) achieved a sustained virologic response 12 weeks after completing therapy (SVR12, considered a cure). In the end, 14 participants were required for the third cohort, because one was incarcerated early on during treatment and another experienced viral rebound at week eight as a result of not adhering to the treatment regimen. Of the other 12 participants, eight, or two-thirds, have achieved an SVR12, while one more participant stopped taking the therapy at week eight but has since achieved an SVR8.
PATENT
WO 2011054834
Scheme 1
GOING TO PRODUCT USING STRUCTURES FROM PATENT
DO NOT MISS OUT synthesis of XIIIa or XIII'a, this is needed in one of side chain
or
MY CONSTRUCTION of 3
Compound 3 was prepared following the procedure reported for the synthesis of compound 1 using intermediate XVIIIb instead of intermediate XVIIIa. see my construction below
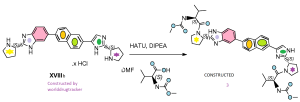
Compound 3. BASE
1H NMR (400 MHz, DMSO-d6) δ ppm 8.34 (2 H, s), 8.21 (1 H, s), 8.19
(1 H, d, J=8.69 Hz), 8.06 - 8.11 (2 H, m), 8.00 (1 H, dd, J=8.88, 1.61 Hz), 7.88 - 7.96
(2 H, m), 7.86 (1 H, d, J=8.48 Hz), 7.32 (1 H, d, J=8.48 Hz), 7.34 (1 H, d, J=8.53 Hz), 5.27 (1 H, dd, J=8.17, 5.33 Hz), 5.17 (1 H, t, J=7.00 Hz), 4.15 (2 H, t, J=7.95 Hz), 3.84
- 3.96 (4 H, m), 3.56 (6 H, s), 2.38 - 2.47 (2 H, m), 1.95 - 2.30 (8 H, m), 0.86 (3 H, d,
J=6.70 Hz), 0.85 (3 H, d, J=6.70 Hz), 0.81 (6 H, d, J=6.63 Hz).
[a] 2°= -148.98 0 (c 0.3336 w/v %, MeOH)
Alternative preparation of compound 3 and the corresponding HC1 salt
N-methoxycarbonyl-L- Valine (3.09 g, 17.7 mmol, 2.1 equiv) was dissolved in dichloro- methane (300 mL). Triethylamine (11.7 mL, 84.1 mmol, 10 equiv) and (l-cyano-2- ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluoro- phosphate were added (7.57 g, 17.7 mmol, 2.1 eq). The reaction mixture was stirred at room temperature for 5 minutes, after which XVIIIb was added (5 g, 8.41 mmol in case x.HCl equals 4 HC1). Stirring was continued for 30 minutes. HC1 in iPrOH (6N) was added to the mixture (until pH = 2), and the resulting mixture was stirred for 5 minutes. The solution was then washed with saturated aqueous sodium carbonate (2 x 200 mL) and once with brine (200 mL). The organic layer was separated, dried on magnesium sulphate and filtrated. After removal of the solvent in vacuum, the obtained residue was further dried in vacuum to afford an orange powder (6.84 g)
The powder was purified by silica gel column chromatography using gradient elution with 0 to 10 % MeOH (7N NH3) in dichloromethane, resulting in compound 3 (2.81 g) as a foam.
Compound 3 was dissolved in iPrOH (40 mL) and HC1 (6N in iPrOH, 10 mL) was added. The volatiles were removed in vacuum. Then, iPrOH (30 mL) was added and the mixture was heated at reflux. The solution was cooled to room temperature and stirred at room temperature for 4 days. tBuOMe (100 mL) was added to the solution, resulting in white precipitation, which was filtered, washed immediately with tBuOMe (3 x 10 mL) under nitrogen atmosphere and dried under vacuum at 40°C. The residue was mixed with acetonitrile and evaporated to dryness (2x). The residue was stirred in acetonitrile (150 mL) and the mixture was sonicated for 10 minutes. The precipitate was filtered under nitrogen atmosphere, washed twice with acetonitrile (50 mL) and dried in vacuum at 40°C, resulting in a slightly yellow powder (4 g).
HCL salt of compound 3:
[a] *° = -110.02 ° (589 nm, 20 °C, c 0.429 w/v%, MeOH)
1H NMR (600 MHz, DIMETHYLFORMAMIDE- y, 280K) δ ppm 0.86 (d, J=6.6 Hz, 6 H), 0.95 (d, J=7.0 Hz, 6 H), 2.03 - 2.20 (m, 2 H), 2.26 - 2.37 (m, 3 H), 2.39 - 2.61 (m, 5 H), 3.61 - 3.63 (m, 6 H), 3.93 - 4.01 (m, 2 H), 4.23 - 4.32 (m, 2 H), 4.32 - 4.39 (m, 2 H), 5.49 (t, J=7.5 Hz, 1 H), 5.52 (dd, J=8.3, 5.3 Hz, 1 H), 7.22 (d, J=8.8 Hz, 1 H), 7.27 (d, J=8.8 Hz, 1 H), 7.98 (d, J=8.6 Hz, 1 H), 8.01 (dd, J=8.6, 1.1 Hz, 1 H), 8.03 (dd, J=8.8, 1.8 Hz, 1 H), 8.09 (d, J=8.8 Hz, 1 H), 8.19 (d, J=8.8 Hz, 1 H), 8.22 (dd, J=8.4, 1.8 Hz, 1 H), 8.25 (s, 1 H), 8.32 (s, 1 H), 8.41 (s, 1 H), 8.88 (s, 1 H).
Anal. Calcd for C42H5oN806 . 2 HCl . 4 H20: C 55.56, H 6.66 , N 12.34. Found: C 55.00, H 6.60, N 12.30
Going reverse.......................
Intermediate XVIIIb
2.8 preparation of intermediate XVIIIb (A=
To a solution of XVIIb (960 mg, 1.48 mmol) in CH2C12 (25mL) was added HCI (5-6 M in isopropanol, 5 mL). The mixture was stirred at room temperature overnight. The solvent was evaporated, the obtained solid was dried in vacuum and used as such in the next step. 2.8a Alternative preparation of intermediate XVIIIb (A=
XVIIb (19.52 g, 30.1 mmol, 1.00 equiv.) was dissolved in dichloromethane (200 mL) and HCI in isopropanol (5-6 N, 300 mL) was added. The reaction mixture was stirred for 1 hour at room temperature. tBuOMe (1000 mL) was added to the suspension and the slurry was stirred at roomtemperature for 30 minutes. The filtered solid was rinced with tBuOMe (2x 100 mL) and dried under vacuum overnight to afford XVIIIb as a powder (15.2 g). 1H NMR (400 MHz, MeOD-d4) δ ppm 2.15 - 2.37 (m, 2 H), 2.37 - 2.52 (m, 2 H), 2.52 - 2.69 (m, 2 H), 2.69 - 2.88 (m, 2 H), 3.56 - 3.71 (m, 4 H), 5.19 - 5.41 (m, 2 H), 7.90 - 8.02 (m, 3 H), 8.05 (dd, J= 8.6, 1.6 Hz, 1 H), 8.10 - 8.25 (m, 4 H), 8.30 (d, J=1.4 Hz, 1 H), 8.47 (d, J=1.2 Hz, 1 H)
INTERMEDIATE XVIIb
2.7 reparation of intermediate XVIIb (A= PG= Boc)
To boronic ester XVIb (1.22 g, 2.26 mmol), bromide Xllla (1072 mg, 3.39 mmol), sodium bicarbonate (380 mg, 4.52 mmol), Pd(dppf)Cl2 (166 mg, 0.226 mmol) in toluene (50 mL), was added water (1 mL). The resulting mixture was heated at reflux overnight. The reaction mixture was filtered, evaporated to dryness and purified by column chromatography by gradient elution with heptane to ethyl acetate. The collected fractions containing the product were pooled and the volatiles were removed under reduced pressure. The residue (960 mg, 65 %) was used as such in the next reaction.
2.7a Alternative preparation of intermediate XVIIb (A= . PG= Boc)
XVIb (10 g, 18.5 mmol), Xlll'a (8.76 g, 24 mmol), NaHC03 (9.32 g, 111 mmol) and Pd(dppf)Cl2 (lg) were stirred in dioxane/water (140 mL, 6/1) under argon. The mixture was heated to 85 °C for 15 hours. Brine (100 mL ) was added and the mixture was extracted with CH2CI2, after drying on MgSC^, filtration and evaporation of the solvent, the residue was purified by column chromotography by gradient elution with CH2CI2 to EtOAc to afford XVIIb (7 g, 58 %).
To a stirred, deoxygenated solution of Vlllb (20.0 g, 45.2 mmol, 1.00 equiv.), Ilia (20.6 g, 49.7 mmol, 1.1 equiv.) and sodium bicarbonate (11.4 g, 136 mmol, 3.0 equiv.) in 1 ,4-dioxane/water (500 mL, 5: 1) under nitrogen, was added l.,.r-Bis(diphenyi~ phosphmo)ferrocene-paiIadium(]I)dichloride dichJoromethane complex (2.50 g, 4.52 mmol, 0.1 equiv.). The mixture was heated at 80°C under argon for 15 hours and cooled to room temperature. The reaction mixture was diluted with dichloromethane (500 mL) and washed with brine (2 x 150 mL) dried on magnesium sulphate; filtered and evaporated to dryness to afford a dark brown foam (43 g). The foam was purified using silicagel column chromatography (gradient elution with 0-6% MeOH in CH2CI2) to afford XVIIb (19.52 g, 65%) as an off-white powder.
INTERMEDIATE XVIb
Bromide XVb (1890 mg, 3.83 mmol), 4,4,4\4\5,5,5\5*-octamethyl-2,2'-bis(l,3,2- dioxaborolane) (2437 mg, 9.59 mmol), KF (390 mg; 6.71 mmol) and (dppf)PdCl2 (281 mg, 0.384 mmol) were dissolved in toluene (50 mL) and heated 3 days at reflux.
The solids were removed by filtration over dicalite and the filtrate was evaporated to dryness on silica. The residue was purified by column chromatography using a heptane to ethylacetate gradient. The fractions containing the product were pooled and the solvent was removed under reduced pressure. The residue (1.22 g, 59 %) was used as such in the next reaction
Under nitrogen, Ilia (25 g, 60.5 mmol), 6-bromonaphthalen-2-yl trifluoromethane- sulfonate (20 g, 56.7 mmol), K3P04 (36.65 g, 173 mmol) and (PPh3)4Pd (717 mg, 0.62 mmol) were stirred in THF (60 mL) and water (15 mL) with the heating mantle at 85 °C (reflux) for 2 hours. CH2CI2 (50 mL) was added and the water layer was separated. The organic layer was dried on MgS04 and after filtration, the filtrate was concentrated resulting in a sticky solid. The residue was purified by column
chromatography (petroleum ether/Ethyl acetate 15/1 to 1/1) to afford XVb (20 g;
40.6 mmol). Compound XVb (1 g, 2.0 mmol), potassium acetate (0.5 g, 5.0 mmol), 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bis(l,3,2-dioxaborolane) (1.29 g, 5.0 mmol), and Pd(dppf)Cl2 (0. lg) were stirred in DMF (15 mL) under argon. The mixture was heated at 60°C for 5 hours. After cooling, CH2CI2 (50 mL) was added and the mixture was washed with saturated NaHC03. The water layer was separated and extracted with CH2CI2. The organic layers were combined and dried on MgSC^. After filtration the solvent was removed and the product was purified by column chromatography (gradient elution with petroleum ether/ethyl acetate 10/1 to 1/1) to give of XVIb (0.7 g,1.3 mmol, 65 %) as light yellow solid.
INTERMEDIATE XVb
2,6-Dibromonaphthalene (6.92 g, 24.2 mmol), boronic ester Ilia (2 g, 4.84 mmol), NaHC03 (813 mg, 9.68 mmol), (dppf)PdCl2(710 mg, 0.968 mmol) were dissolved in toluene (75 mL). Water (1 mL) was added and the mixture was heated for 7 hours at reflux. The solids were removed by filtration over dicalite and the filtrate was evaporated to dryness on silica. The residue was purified by column chromatography by gradient elution with heptane to ethylacetate. The appropriate fractions were pooled and the solvent was removed under reduced pressure. The residue (1.89 g, 79 %) was used as such in the next step.
1.2 Preparation of intermediate IIIa (PG= Boc)
To a mixture of Ila (200 g, 546 mmol), potassium acetate (160.8 g, 1.64 mol) and 4,4,4*,4*,5,5,5*,5*-octamethyl-2,2,-bis(l,3,2-dioxaborolane) (416 g, 1.64 mol) in DMF (3L) was added Pd(dppf)Cl2 (20 g) under nitrogen gas. The reaction mixture was stirred at 85°C for 15 hours. The mixture was diluted with ethyl acetate, washed with water and brine, dried over magnesium sulfate, the solids removed by filtration, and the solvents of the filtrate were removed under reduced pressure. The residue was purified by silica column chromatography (petroleum ether : ethyl acetate 10: 1 to 2: 1) to afford 125 g of Ilia as a white solid (contains 15% of boronic acid).
INT IIa
1.1 preparation of intermediate Ila (PG= Boc; X= Br)
Ma
To a solution of Boc-Z-Proline (2669 mg, 12.4 mmol) in pyridine/DMF (30 mL, 1/1) was added di(lH-imidazol-l-yl)ketone (2205 mg, 13.6 mmol). The mixture was stirred at 45°C for 2 hours. 4-bromobenzene-l,2-diamine (2319 mg, 12.4 mmol) was added and the mixture was stirred at ambient temperature overnight. The solvent was removed and the residue heated in acetic acid (15 mL) at 100°C for 30 minutes. After
concentration of the residue, the mixture was partitioned between ethyl acetate and a saturated sodium bicarbonate solution. The organic phase was separated and washed with water, after drying over Na2SC"4, the mixture was filtrated and the filtrate was concentrated in vacuum. The obtained residue was purified by flash chromatography using CH2Cl2/EtOAc 90/10 to 50/50, resulting in compound Ila (3.146 g, 69 %).
DO NOT MISS OUT synthesis of XIIIa or XIII'a, this is needed in one of side chain
2.1 preparation of L-boc-prolinol
Borane-methyl sulfide complex (180 mL, 1.80 mol) was added dropwise to a solution of N-Boc- L-Proline (300 g, 1.39 mol) in anhydrous THF (3.0 L) which was cooled to 0°C. When gas evolution ceased, the ice bath was removed and the solution was stirred at 10°C for 18 hours. Thin layer chromatography (TLC) showed that no starting material remained and that the desired product was formed. The solution was cooled to 0°C and methanol (2.4 L) was slowly added. The solvents were removed under reduced pressure. The residue was reconstituted in dichloromethane (1 L), washed with
NaHC03 (500 mL, saturated, aqueous) and brine (500 mL), dried over MgS04, the solids were removed via filtration, and the solvents of the filtrate were removed under reduced pressure to afford a white solid, 260 g (93%), used in the next step without further purification.
2.2 preparation of Z-boc-prolinal
To a solution of Z-boc-prolinol (100 g, 500 mmol) in CH2CI2 (1.5 L) at 0°C were added successively, under vigorous stirring, 2,2,6,6-tetramethylpiperidine-l-oxyl (TEMPO; 1.56 g, 10 mmol) and NaBr (5.14 g, 50 mmol). To the resulting mixture was added dropwise a solution of NaHC03 (6.3 g, 75 mmol) and 6% NaCIO in active chlorine (750 mL, 750 mmol) at 0°C over a period of 1 hour. TLC showed no starting material remained and that the desired product was formed. The mixture was rapidly extracted with dichloromethane (2 x 1.5 L). The organic layers were combined, washed with NaHS04 (10%, 1 L) and KI (4%, 200 mL), then with Na2S203 (10%, 1 L) and brine (1.5 L), dried over MgS04, the solids were removed via filtration, and the solvents evaporated to afford a yellow oil, Z-boc-prolinal, (89 g, 92%>), used in the next step without further purification.
2.3 preparation of intermediate XXIV
ammonia
XXIV
Aqueous ammonia (25~28%>, 200 mL) was added dropwise to a solution of L-boc- prolinal (89 g, 0.44 mol) and glyoxal (183 mL of 40% in water) in methanol (1 L). The reaction mixture was sealed and reacted at 10°C. After 16 hours, additional glyoxal (20 mL) and aqueous ammonia (20 mL) were added and reacted for an additional 6 hours. The solvents were removed under reduced pressure, and the crude was reconstituted in ethyl acetate (1.0 L), washed with water and brine, dried over MgSC^, the solids were removed via filtration and the solvents were removed under reduced pressure. The crude was purified by column chromatography (silica gel, dichloromethane to methanol/dichloromethane 1 :70) to obtain 73 g (70%) intermediate XXIV as a white solid.
1H NMR: (CD3OD 400 MHz) δ 6.95 (s, 2H), 4.82-4.94 (m, 1H), 3.60-3.70 (m, 1H), 3.41-3.50 (m, 1H), 2.20-2.39 (m, 1H), 1.91-2.03 (m, 3H), 1.47 (s, 3H), 1.25 (s, 6H)
2.4 preparation of intermediate XHIa (PG= Boc)
XXIV Xllla
N-Bromosuccinimide (47.2 g, 0.26 mol) was added portion wise over 1 hour to a cooled (ice-ethanol bath, -10 °C) solution of XXIV (63.0 g, 0.26 mol) in CH2C12 (1.5 L) and stirred at similar temperature for 2 hours. The reaction mixture was concentrated in vacuum and the residue was purification by preparatory HPLC to provide 25.3 g (30%) of Xllla as a pale yellow solid.
1H NMR: CD3OD 400Mhz
δ 6.99-7.03 (s,lH), 4.77-4.90 (m, 1H), 3.61-3.68 (m, 1H), 3.42-3.50 (m, 1H), 2.20-2.39 (m, 1H), 1.89-2.05 (m, 3H), 1.47 (s, 3H), 1.27 (s, 6H).
2.4a preparation of intermediate XHI'a (PG= Boc)
To a solution of iodine (43.3 g, 170.5 mmol, 2 eq) in chloroform (210 mL) in a round bottomed flask (1L) a suspension of XXIV (20 g, 84.3 mmol) in an aqueous NaOH solution (2M, 210 mL) was added. The mixture was stirred at room temperature for 15 hours. To the resulting reaction mixture was added a saturated aqueous Na2S2C"3 solution (100 mL) and the organic layer was separated. The aqueous layer was extracted with chloroform (4x 150 mL). The organic layers were combined, washed with water and dried on magnesium sulphate. The solids were filtered and the solution was evaporated to dryness to afford diiodide (38.61 g, 89 %).
The above obtained intermediate diiodide (2.24 g, 4.58 mmol) and sodium sulfite (4.82 g, 38 mmol) were placed in a round bottomed flask (100 mL) and suspended in 30% EtOH/water (80 mL). The resulting mixture was refluxed for 40 hours. The solvent was removed and after addition of H20 (20 mL), the mixture was stirred at room temperature overnight. The solids were filtered, washed with water and dried in a vacuum oven to afford compound XHI'a (1.024 g, 61 %).
1H NMR (400 MHz, DMSO-d6) δ ppm 1.16 and 1.38 (2x br. s., 9 H), 1.68 - 2.02 (m, 3 H), 2.02 - 2.27 (m, 1 H), 3.18 - 3.38 (m, 1 H), 3.38 - 3.59 (m, 1 H), 4.53 - 4.88 (m, 1 H), 6.81 (m, -0.1 H), 7.05 - 7.28 (m, -0.9 H), 11.90 - 12.20 (m, -0.9 H), 12.22 - 12.40 (m, -0.1 H)
PATENT
WO 2011149856
1st scheme

IN ABOVE SCHEME CONVERSION OF f to g N-methoxycarbonyl-L-Val-OH is used,
USE R =H IN LAST STEP TO GET RAVIDASVIR
EXAMPLE 1 - Synthesis of compounds of Formula lie
Scheme 1-1 describes preparation of target molecules and their analogs with symmetrical and non-symmetrical functionalized ends.
[0341] Step a. To a solution of 2-bromonaphthane a (62.0 g, 300 mmol) in DCM (1 L) was added A1C13 (44.0 g, 330 mmol) and 2-chloroacetyl chloride (34.0 g, 330 mmol) at 0 °C. The reaction mixture was stirred at 0 °C for 1 h and then H20 added (500 mL) and extracted. The organic layer was washed with H20, dried over anhydrous Na2S04, evaporated under reduced pressure to give 80 g crude product, which was purified by re-crystallization from 10% EtOAc- hexane (v/v) to yield b (28 g, 36% yield) as a white solid: JH NMR (500 MHz, CDC13) δ 8.44 (s, 1H), 8.07 (s, 1H), 8.04 (d, J= 11.0 Hz, 1H), 7.84 (d, J= 8.5 Hz, 2H), 7.66 (d, J= 8.5 Hz, 1H), 4.81 (s, 2H) ppm; LCMS (ESI) m/z 282.9 (M + H)+.
Step b. To a solution of b (28.0 g, 100 mmol) in DCM (500 mL) was added N-Boc- L-Pro-OH (24.7 g, 115 mmol) and Et3N (70.0 mL, 500 mmol) and the mixture was stirred at rt for 2 h. The mixture was concentrated under reduced pressure to afford crude c which was used for the next step without further purification. LC-MS (ESI) m/z 462.1 (M + H)+.
Step c. To a solution of c (46.0 g, 100 mmol) in toluene (500 mL) was added
NH4OAc (77 g, 1.0 mol) and the mixture was stirred at 110 °C overnight, and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (petroleum ether/EtOAc l :l(v/v)) to afford d (30 g, 68% yield) as a yellow solid: LC-MS (ESI) m/z 442 A (M + H)+.
Step d. To a solution of d (10.0 g, 23.0 mmol) in anhydrous DME (200 mL) and equal molar of boronate e was added PPh3 (1.2 g, 4.6 mmol), Pd(PPh3)4 (1.6 g, 2.3 mmol), and 2.0 M Na2C03 solution. The mixture was refluxed under argon overnight. The organic solvent was removed under reduced pressure and the residue was treated with H20, extracted with EtOAc (2 x 200 mL). The combined organic phase was dried, filtered, and concentrated in vacuo to give a residue, which was purified by silica gel column chromatography (petroleum
ether/EtOAc 3: l(v/v)) to afford f (10 g, 96% yield) as a yellow solid. LC-MS (ESI): m/z 709.3 (M+H)+.
Step e. To a stirred solution of f (150 mg, 0.29 mmol) in dioxane (3 mL) was added 4.0 N HCl in dioxane (3 mL) dropwise. The mixture was stirred at rt for 4 h, and then
concentrated to yield a yellowish solid (134 mg), which was used directly for the next step. The residue (134 mg, 0.290 mmol) was suspended in THF (5 mL) and DIPEA (0.32 mL) was added and followed by addition of N-methoxycarbonyl-L-Val-OH (151 mg, 0.860 mmol). After stirring for 15 min, HATU (328 mg, 0.860 mmol) was added and the mixture was stirred at rt for another 2 h and then concentrated. The residue was purified by prep-HPLC to obtain g (40 mg, 19% yield).
2nd scheme

SCHEME SIMILAR UPTO PENULTIMATE STEP
Note 9 is not final product pl ignore it
Step a. Referring to Scheme 1-2, to a solution of compound 3 (2.0 g, 4.5 mmol) in dioxane (25 mL) was added 4.0 N HCl in dioxane (25 mL). After stirring at rt for 4 h, the reaction mixture was concentrated and the residue was dried in vacuo to give a yellowish solid (2.1 g), which was used directly for the next step without further purification.
[0347] Step b. To the residue of step a (4.5mmol) was added DMF (25 mL), followed by adding HATU (2.1 g, 5.4 mmol), DIPEA (3.7 mL, 22.5 mmol) and N-methyl carbamate-L-valine (945 mg, 5.4 mmol). After stirring at rt for 15 min, the reaction mixture was added slowly to H20 (400 mL). A white solid precipitated was filtered and dried to give compound 6 (2.2 g, 98% yield). LC-MS (ESI): m/z 499.1 (M+H)+.
[0348] Step c. To a mixture of compound 6 (800 mg, 1.6 mmol), compound 7 (718 mg, 1.6 mmol), and NaHC03 (480 mg, 5.7 mmol) in 1 ,2-dimethoxyethane (15mL) and H20 (5mL) was added Pd(dppf)Cl2 (59 mg, 0.08 mmol). After stirring at 80°C overnight under an atmosphere of N2, the reaction mixture was concentrated. The residue was partitioned between 20%
methanol/CHCl3 (100 mL) and H20 (100 mL). The organic phase was separated and the aqueous phase was extracted with 20% methanol/CHCl3 (100 mL) again. The combined organic phase was consequently washed with brine, dried with anhydrous Na2S04, filtered, and concentrated. The residue was purified by silica gel column chromatography (Petroleum
ether/EtOAc=15: l(v/v)) to give compound 8 (1.0 g, 85% yield) as a yellow solid. LC-MS (ESI): m/z 732.4 (M+H)+.
Step d. To a solution of compound 8 (200 mg, 0.27 mmol) in dioxane (3.0 mL) was added 4 N HCl in dioxane (3.0 mL). After stirring at rt for 2 h, the reaction mixture was concentrated and the residue was dried in vacuo to give an HCl salt in quantitative yield, which was used directly for the next step without further purification..............CAN BE USED AS PRECURSOR FOR RAVIDASVIR UPTO THIS POINT
CAUTION SIMILAR BUT NOT SAME........Step e. To a solution of the salt (0.27 mmol) in DMF (5.0 mL) was added DIPEA (0.47mL, 2.7 mmol), followed by adding N,N-dimethyl-D-phenyl glycine (59 mg, 0.33 mmol) and HATU (125 mg, 0.33 mmol). After stirring at rt for lh, the reaction mixture was partitioned between H20 and DCM. The organic phase was washed successively with H20 and brine, dried with anhydrous Na2S04, filtered, and concentrated. The residue was purified by prep-HPLC to give compound 9........CAUTION SIMILAR BUT NOT SAME. LC-MS (ESI): m/z 793.4 (M+H)+.
3rd scheme

SCHEME SIMILAR UPTO PENULTIMATE STEP
15 NOT THE COMPD PL IGNORE IT IF YOU NEED RAVIDASVIR
Step a. To a mixture of compound 3 (3.2 g, 7.2 mmol), bis(pinacolato)diboron (3.86 g, 15.2 mmol), and KOAc (1.85g, 18.8mmol) in 1,4-dioxane (100 mL) was added Pd(dppf)Cl2 (440 mg, 0.6 mmol). After stirring at 80 °C for 3 h under an atmosphere of N2, the reaction mixture was concentrated. The residue was purified with silica gel column chromatography (Petroleum ether/EtOAc=2/l(v/v)) to give compound 11 (2.8 g, 80% yield) as a white solid. LC- MS (ESI): m/z 490.3 (M+H)+.
[0352] Step b. To a mixture of compound 11 (626 mg, 1.27 mmol), compound 12 (570 mg, 1.27 mmol), and NaHC03 (420 mg, 4.99 mmol) in 1, 2-dimethoxyethane (30 mL) and H20 (10 mL) was added Pd(dppf)Cl2 (139 mg, 0.19 mmol). After stirring at 80°C overnight under an atmosphere of N2, the reaction mixture was concentrated. The residue was partitioned between 20% methanol/CHCl3 (100 mL) and H20 (100 mL). The aqueous phase was extracted with 20% methanol/CHCl3 (100 mL) again. The combined organic phase was consequently washed with brine, dried with anhydrous Na2S04, filtered, and concentrated. The residue was purified by silica gel column chromatography (Petroleum ether/EtOAc=2/l(v/v)) to give compound 13 (635 mg, 68% yield) as a yellow solid. LC-MS (ESI): m/z 732.4 (M+H)+.
Step c. To a solution of compound 13 (200 mg, 0.27 mmol) in dioxane (3.0 mL) was added 4 N HC1 in dioxane (3.0 mL). After stirring at rt for 2 h, the reaction mixture was concentrated and the residue was dried in vacuo to yield the HC1 salt of compound 14 in quantitative yield, which was used directly for the next step without further purification.....CAN BE USED AS PRECURSOR FOR RAVIDASVIR UPTO THIS POINT
CAUTION SIMILAR BUT NOT SAME.........Step d. To a solution of the salt (0.27 mmol) in DMF (5.0 mL) was added DIPEA (0.47 mL, 2.7 mmol), followed by adding N,N-dimethyl-D-phenyl glycine (59 mg, 0.33 mmol) and HATU (125 mg, 0.33 mmol). After stirring at rt for lh, the reaction mixture was partitioned between H20 and DCM. The organic phase was consequently washed with H20 and brine, dried with anhydrous Na2S04, filtered, and concentrated. The residue was purified by prep-HPLC to give compound 15..CAUTION SIMILAR BUT NOT SAME. LC-MS (ESI): m/z 793.4 (M+H)+.
4 th scheme

SCHEME SIMILAR UPTO PENULTIMATE STEP
5 NOT THE COMPD, PL IGNORE IT IF YOU NEED RAVIDASVIR
4 CAN BE USED AS PRECURSOR FOR RAVIDASVIR UPTO THIS POINT
scheme ........CAUTION SIMILAR BUT NOT SAME
EXAMPLE 2 - Synthesis of compounds of Formula Hie
Step a. Referring to Scheme 2-1, to a mixture of compound 1 (5.05 g, 13.8 mmol), bis(pinacolato)diboron (7.1 g, 27.9 mmol), and KOAc (3.2 g, 32.5 mmol) in 1,4-dioxane (100 mL) was added Pd(dppf)Cl2 (400 mg, 0.5 mmol). After stirring at 80 °C for 3 h under an atmosphere of N2, the reaction mixture was concentrated. The residue was purified by silica gel column chromatography (Petroleum ether/EtOAc=2/l(v/v)) to give compound 2 (3.0 g, 53% yield) as a gray solid. LC-MS (ESI): m/z 414.2 (M+H)+.
Step b. To a mixture of compound 2 (522 mg, 1.26 mmol), compound 3 (500 mg, 1.13 mmol), and NaHC03 (333 mg, 3.96 mmol) in 1, 2-dimethoxyethane (30 mL) and H20 (10 mL) was added Pd(dppf)Cl2 (74 mg, 0.1 mmol). After stirring at 80°C overnight under an atmosphere of N2, the reaction mixture was concentrated. The residue was partitioned between 20% methanol/CHCl3 (100 mL) and H20 (100 mL). The organic phase was separated and the aqueous phase was extracted with 20% methanol/CHCl3 (100 mL) again. The combined organic phase was consequently washed with brine, dried with anhydrous Na2S04, filtered, and concentrated. The residue was purified by silica gel column chromatography (DCM/MeOH=50:l (v/v)) to give compound 4 (450 mg, 55% yield) as a yellow solid. LC-MS (ESI): m/z 649.3 (M+H)+.
Step c. To a stirred solution of compound 4 (160 mg, 0.25 mmol) in dioxane (2.0 mL) was added 4N HCl in dioxane (2.0 mL). After stirring at rt for 3h, the reaction mixture was concentrated and the residue was dried in vacuo to give an HCl salt in quantitative yield, which was used directly for the next step without further purification.4 CAN BE USED AS PRECURSOR FOR RAVIDASVIR UPTO THIS POINT
SCHEME SIMILAR UPTO PENULTIMATE STEP
5 NOT THE COMPD, PL IGNORE IT IF YOU NEED RAVIDASVIR
scheme ........CAUTION SIMILAR BUT NOT SAME
5 th scheme
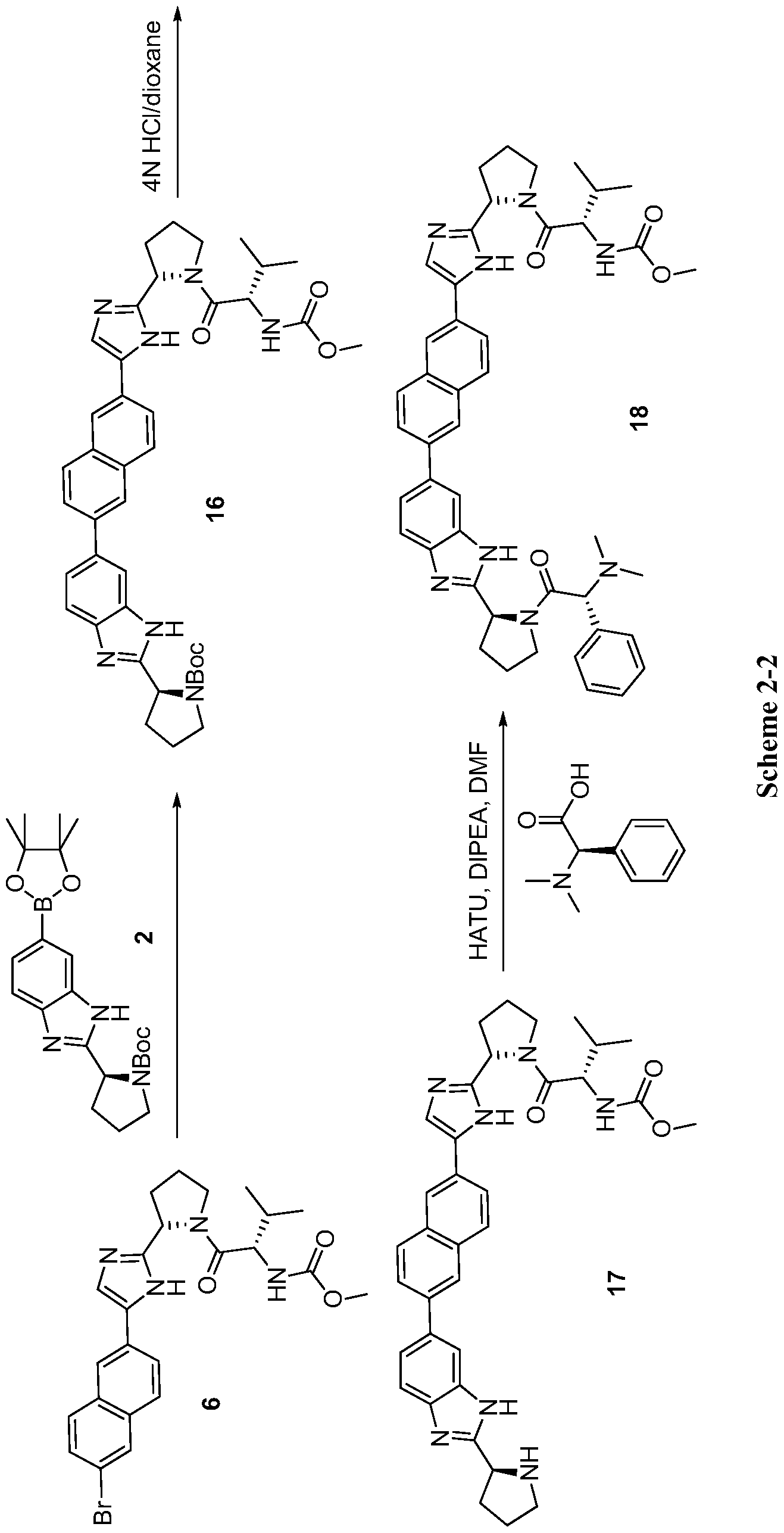
SCHEME SIMILAR UPTO PENULTIMATE STEP
18NOT THE COMPD, PL IGNORE IT IF YOU NEED RAVIDASVIR
17 CAN BE USED AS PRECURSOR FOR RAVIDASVIR UPTO THIS POINT
scheme ........CAUTION SIMILAR BUT NOT SAME
Step a. Referring to Scheme 2-2, to a mixture of compound 2 (1.16 g, 2.32 mmol), compound 6 (1.40 g, 3.39 mmol), and NaHC03 (823 mg, 9.8 mmol) in 1, 2-dimethoxyethane (30 mL) and H20 (10 mL) was added Pd(dppf)Cl2 (103 mg, 0.14 mmol). After stirring at 80 °C over night under an atmosphere of N2, the reaction mixture was concentrated. The residue was partitioned between 20% methanol/CHCl3 (150 mL) and H20 (150 mL). The aqueous phase was extracted with 20% methanol/CHCl3 (150 mL) again. The combined organic phase was consequently washed with brine, dried with anhydrous Na2S04, filtered, and concentrated. The residue was purified by silica gel column chromatography (Petroleum ether/acetone=1.5/l (v/v)) to give compound 16 (1.32g, 80% yield) as a yellow solid. LC-MS (ESI): m/z 706.4 (M + H)+.
tep b. To a solution of compound 16 (200 mg, 0.28 mmol) in dioxane (3.0 mL) was added 4 N HC1 in dioxane (3.0 mL). After stirring at rt for 2 h, the reaction mixture was concentrated and the residue was dried in vacuo to give the HC1 salt of compound 17 in quantitative yield, which was used directly for the next step.......17 CAN BE USED AS PRECURSOR FOR RAVIDASVIR UPTO THIS POINT
6 th scheme
 scheme 2-3
scheme 2-3
SCHEME SIMILAR UPTO PENULTIMATE STEP
22NOT THE COMPD, PL IGNORE IT IF YOU NEED RAVIDASVIR
21 CAN BE USED AS PRECURSOR FOR RAVIDASVIR UPTO THIS POINT
scheme ........CAUTION SIMILAR BUT NOT SAME
Scheme 2-3
Step a. Referring to Scheme 2-3, to a solution of compound 1 (4.0 g, 10.9 mmol) in dioxane (40 mL) was added 4 N HC1 in dioxane (40 mL). After stirring at rt overnight, the reaction mixture was concentrated. The residue was washed with DCM, filtered, and dried in vacuo to afford a hydrochloride salt in quantitative yield, which was used for the next step without further purification.
Step b. To a solution of the salt (10.9 mmol) in DMF (30 mL) was added DIPEA (5.8 mL, 33.0 mmol), followed by adding N-methoxycarbonyl-L-valine (2.1 g, 12.1 mmol) and HATU (4.6 g, 12.1 mmol). After stirring at rt for lh, the reaction mixture was partitioned between H20 and DCM. The organic phase was consequently washed with H20 and brine, dried with anhydrous Na2S04, filtered, and concentrated. The residue was purified by silica gel column chromatography (DCM/Petroleum ether=4/l (v/v)) to give compound 19 (3.0 g, 65% yield). LC- MS (ESI): m/z 423.1 (M+H)+.
Step c. To a mixture of compound 11 (800 mg, 1.9 mmol), compound 19 (700 mg, 1.7 mmol), and NaHC03 (561 mg, 6.6 mmol) in 1, 2-dimethoxyethane (60 mL) and H20 (20 mL) was added Pd(dppf)Cl2 (183 mg, 0.25 mmol). After stirring at 80 °C overnight under an atmosphere of N2, the reaction mixture was concentrated. The residue was then partitioned between 20% methanol/CHCl3 (100 mL) and H20 (100 mL). The aqueous phase was extracted with 20% methanol/CHCl3(100 mL) again. The combined organic phase was consequently washed with brine, dried with Na2S04, filtered, and concentrated. The residue was purified by silica gel column chromatography (Petroleum ether/EtOAc=2/l(v/v)) to give compound 20 (600 mg, 52% yield) as a yellow solid. LC-MS (ESI): m/z 706.4 (M+H)+.
Step d. To a solution of compound 20 (200 mg, 0.28 mmol) in dioxane (3.0 mL) was added 4N HC1 in dioxane (3.0 mL). After stirring at rt for 2h, the reaction mixture was concentrated and the residue was dried in vacuo to yield the HC1 salt of compound 21 in quantitative yield, which was used directly for the next step without further purification.
21 CAN BE USED AS PRECURSOR FOR RAVIDASVIR UPTO THIS POINT
7 th scheme

Scheme 6-2
SCHEME SIMILAR UPTO n-2 STEP in above scheme
84, 85 NOT THE COMPD, PL IGNORE IT IF YOU NEED RAVIDASVIR
83 CAN BE USED AS early PRECURSOR FOR RAVIDASVIR UPTO THIS POINT
scheme ........CAUTION SIMILAR BUT NOT SAME
Step a. Referring to Scheme 6-2, a solution of compound 78 (50.0 g, 0.30 mol) in THF (500 mL) and H20 (500 mL) was added K2C03 (83 g, 0.60 mol) and (Boc)20 (73. Og, 0.330 mol). After stirring at rt overnight, the reaction mixture was concentrated and the residue was extracted with EtOAc (250 mL x 3). The extracts were combined, washed with brine, and dried with anhydrous Na2S04. The solvent was removed and the residue was dried in vacuo to give crude compound 78 (62 g), which was used for the next step without further purification. LC-MS (ESI) m/z 230.1 (M + H)+.
[0453] Step b. To a solution of compound 78 (60.0 g, 260 mmol) in EtOH (1 L) was slowly added NaBH4 (50.0 g, 1.30 mol) at rt. After stirring at rt overnight, the reaction was quenched by adding acetone (10 mL). The resulting mixture was concentrated and the residue was diluted with EtOAc (500 mL). The mixture was washed with brined and dried in vacuo. The solvent was removed and the residue was purified by silica gel column chromatography (Petroleum ether/EtOAc = 1/1 (v/v)) to give compound 79 (42.0 g, 80% yield) as a white solid. LC-MS (ESI) m/z 202 A (M + H)+.
[0454] Step c. To a solution of compound 79 (30.0 g, 150 mmol) and DMSO (35.0 g, 450 mmol) in DCM (1 L) was added oxalyl chloride (28.0 g, 220 mmol) at -78 °C. After stirring at - 78 °C for 4 h, the reaction mixture was added Et3N (60.0 g, 600 mol) and the resulting mixture was stirred for another 1 h at -78 °C. Subsequently, the reaction was quenched by adding H20. The organic layer was separated and the aqueous layer was extracted with DCM (200mL x 2). The extracts were combined, washed with brine, and dried with Na2S04. The solvent was removed and the residue was dried in vacuo to give crude compound 80 (22.0 g) as a colorless oil, which was used immediately without further purification. LC-MS (ESI) m/z 200.1 (M + H)+.
[0455] Step d. A mixture of compound 80 (7.7 g, 38.5 mmol), 6-bromopyridine-2,3-diamine (8.0 g, 42.8 mmol) (PCT Intl. Appl. WO 2008021851) , and iodine (1.08 g, 4.28 mmol) in AcOH (30 mL) was stirred at rt overnight. The reaction mixture was neutralized by adding saturated aqueous NaHC03. The resulting mixture was extracted with EtOAc (200 mL x 3). The extracts were combined, washed with brine, and dried with anhydrous Na2S04. The solvent was removed and the residue was purified by silica gel column chromatography (DCM/MeOH = 80/1 (v/v)) to give compound 81 (7.8 g, 55% yield). LC-MS (ESI) m/z 367.1 (M + H)+.
[0456] Step e. A mixture of compound 82 (10.0 g, 20.1 mmol), bis(pinacolato)diboron (7.65 g, 30.1 mmol), potassium acetate (6.89 g, 70.3 mmol), and Pd(dppf)Cl2-CH2Cl2 (886 mg, 1.0 mmol) in 1,4-dioxane (200 mL) was stirred at 80 °C for 3 h under an atmosphere of N2. The reaction mixture was filtered through CELITE™ 545 and the filtered cake was washed with EtOAc (200 mL x 3). The filtrate was washed with brine and dried with anhydrous Na2S04. The solvent was removed and the residue was purified by silica gel column chromatography
(DCM/MeOH = 50/1 (v/v)) to give compound 83 (9.8 g, 89% yield) as a white solid: LC-MS (ESI) m/z 547.3 (M + H)+.83 CAN BE USED AS early PRECURSOR FOR RAVIDASVIR UPTO THIS POINT
PATENT
CN 102796084
Step One: Formula (2) compounds strokes trichloride catalyst (AlCl3), chloroacetyl chloride (2-chloroacetylchloride) at room temperature to obtain a compound of formula (3),
(3);
wherein the reaction temperature is room temperature, the solvent is methylene chloride. Material I (i.e., formula (2) compound) and chloroacetyl chloride (2-chloroacetyl chloride) was slowly added, higher yields can be obtained.(3) The compound was recrystallized from ether to obtain.
In the present embodiment, the 20.5 g of formula (2) compound (0. Imol) and 26.2 g AlCl3 (0.2mol) was added to 200ml of dichloromethane, cooled to room temperature, stirring speed slowly was added 13.4 g of chloroacetyl chloride (I. 2mol), within three hours after the addition and then mixed by stirring maintained at room temperature for 3 hours. Was slowly added 50 ml of ice water, the precipitate was collected by filtration. The filter cake was washed with 10 ml of water and 10 ml petroleum ether (twice). The filtrate and the organic layer together with 50 ml of dichloromethane and extracted twice with 50 ml brine and then paint extraction solution, the extract was dried over magnesium sulfate, the solution was removed, the solid with 100 ml of diethyl ether and recrystallized to afford 20g (71% yield compounds) of formula (3).
Step II: Formula (3) with a compound of formula (4) compound under acidic conditions and chloroform (CCl3H) heating the reaction, and the reaction system reached reflux to give a compound of formula (5),
(5);
[0042] wherein, the formula (3) with a compound of formula (4) compound in acetonitrile (chloroform (CCl3H), the reaction system must be reached reflux, and must be reacted under acidic conditions to give the compound of formula (5). [0043] In this embodiment, the compound (3) (0. Imol) 28. 2 克 formula and the compound (4) (0. Imol) 21. 5 克 style with 3 g of trifluoroacetic acid was added to 200 ml of chloroform, in was stirred at reflux under nitrogen for 17 hours. After cooling to room temperature, spin-dry, to give 46. I g of a yellow solid of formula (5) compound (99% yield).
Step three: (5) the compound obtained in toluene (toluene) and ammonium acetate (NH4OAc) reflux (6) of
Thereof,
Compound of formula (5) is ammonium acetate with toluene under reflux conditions for ring closure.
In the present embodiment, the compound (0. Imol) and 10 g of ammonium acetate (NH4OAc) was added 46. I g of formula (5) to IJ 200ml of toluene, heated under reflux for 3 hours with stirring. Was slowly added 50 ml of ice water, filtered, washed with 100 ml of toluene and extracted twice with 50 ml brine and then paint extraction solution, the extract was dried over magnesium sulfate, the solution was removed, the solid with 100 ml of diethyl ether and recrystallized to afford 40g (89% compound yield) of the formula (6).
Step Four: (6) compound in the catalyst and the associated button pinacolato ester (Bis (pinacolato) diboron) reacting a compound of formula (7),
wherein, Pd (dppf) 2Cl2 can be replaced by another of a palladium catalyst, a palladium catalyst with the other, the same effect.
In the present embodiment, 44 g of the compound of formula (6) (0. Imol) and 3 g Pd (dppf) 2C12,25. 4 克 United pinacolato ester (0. Imol) and 8.4 g of sodium bicarbonate (0. Imol) was added to a 200 ml I. 4- dioxane, stirred at reflux for 24 hours. Diatomaceous earth filtration, spin dry. Spin-dry 100 ml of ethyl acetate dissolved. Anhydrous magnesium sulfate and spin dry. Recrystallization from ether to yield 40 g (82% yield) of a yellow solid of formula (7) compound.
Step Five: formula (7) under palladium catalyst compound and the compound (8) obtained by reacting the compound of formula (9),
wherein, Pd (dppf) 2Cl2 can be replaced by another of a palladium catalyst, a palladium catalyst with the other, the same effect.
In the present embodiment, 48.9 g of the compound of formula (7) (0. Imol) and 3 g Pd compound (8) (0. Imol) (dppf) 2C12,41. 3 and 8 克 style. 4 g of sodium hydrogen carbonate (0. Imol) was added to a 200 ml I. 4- dioxane, stirred at reflux for 24 hours. Diatomaceous earth filtration, spin dry. Spin-dry 100 ml of ethyl acetate dissolved.Anhydrous magnesium sulfate and spin dry. Recrystallized from ether to give compound 55 g (85% yield) of a yellow solid of formula (9).
[0056] Step Six: formula (9) compound deprotected under acidic conditions to give a compound of formula (10),
[0057]
In the present embodiment, the 64.8 grams of formula (9) compound (0. Imol) was added to 100 ml I. 4_ dioxane was stirred, 100 ml of 5M / L of I under nitrogen 4- dioxane solution of hydrochloric acid. Spin-dry for 24 hours later, get 52. I g of pale yellow solid formula (10) compound (99% yield).
Step 7: Formula (10) with a compound (11) in a condensing agent is 2- (7-azo BTA) -N, N, N ', N'- tetramethyluronium hexafluorophosphate phosphate (HATU) under condensation reaction conditions to give the final product compound C0S-101, i.e. the compound of formula (I):
In the present embodiment, the compound of formula 52. I g of (10) (0. Imol) was added to a 200 ml N, N- dimethylformamide (DMF) cooled to 0 ° with stirring, in a nitrogen atmosphere was added 20.2 g of triethylamine (0. 2mol) 0 After 10 minutes of stirring, was added 19 g of formula (11) compound (0. Ilmol) was added followed by 26 g HATU (0. 2mol), stirred at room temperature for 32 hours . Was slowly added 50 ml of ice water, the precipitate was collected by filtration. The filter cake was washed with 10 ml of water and 50 ml dichloromethane twice.Together with the filtrate and the organic layer was extracted 2 times 50 ml of dichloromethane, and then washed with 50 ml brine solution, the extract was dried over magnesium sulfate, the solution was removed, solid was recrystallized from 100 ml of ethanol, to give 50g (66% yield) The pale yellow compound C0S-101.
In summary this compound on C0S-101 non-structural protein 5A inhibitor, or a pharmaceutically acceptable salt thereof, the treatment of hepatitis C active substance. A compound of formula (3) Friedel-Crafts reaction occurs directly from 2-bromo-naphthalene chloride and chlorine. A compound of formula (3) with a compound of formula (4) condensing a compound of formula (5). The compound of formula (5) self-condensation of a compound of formula (6). Of formula (6) is reacted with boronic acid pinacol ester linking reaction of the compound of formula (7). A compound of formula (7) with a compound of formula (8) coupling reaction of a compound of formula (9). Off compound under acidic conditions (9) protect the compound of formula (10) and formula (10) compound condensation of the final product C0S-101, method of operation of the invention is simple, mild conditions, process maturity, yield and high purity suitable for industrial production.
PATENT
WO 2013123092

Scheme 3
3-3 2HCI salt
Step 1. Referring to Scheme 3, compounds l-5a (1.3 kg , 1.0 eq.), 2-2a (975.0 g, 1.0 eq.), NaHCOs (860.0 g, 3.80 eq.), Pd(dppf)Cl2 (121.7 g, 0.05 eq.), purified water (5.2 L, 4.0 volume) and 1 ,2-dimethoxy ethane (DME) (24.7 L, 19.0 volume) were charged into a 50.0 L 4-necked round bottom flask under argon atmosphere. After being degassed using argon for a period of 30 min, the reaction mass was slowly heated to ~ 80 °C and stirred at this temperature for 12 - 14 hrs. HPLC analysis indicated that > 97% of compound 2-2a was consumed. Next, the reaction mass was concentrated to completely remove DME under vacuum (600 mmHg) at 40 - 45 °C and the residue was diluted with 20% (v/v) MeOH in DCM (13.0 L , 10 volume) and purified water (13.0 L, 10.0 volume) with stirring. The organic layer was separated and the aqueous layer was extracted with 20% (v/v) MeOH in DCM (6.5 L x 2, 10.0 volume). The combined organic extracts were washed twice with water (6.5 L x 2, 10.0 volume) and once with saturated brine (6.5 L, 5.0 volume) and dried over anhydrous Na2S04. The solvent was removed under vacuum (600 mmHg) and the residue was purified by flash column chromatography using silica gel with hexanes/EtOAc as eluent to give compound 3-1 (1.0 kg, 63% yield) as off white solid with a purity of > 98.0%> determined by HPLC analysis. LC-MS (ESI): m/z 649.3 [M + H]+. 1H NMR (400 MHz, d6- DMSO): δ 12.26 - 12.36 (m, 1H), 11.88 - 11.95 (m, 1H), 8.23 (s, 1H), 8.11 (s, 1H), 7.91 (m, 3H), 7.85 - 7.87 (m, 2H), 7.51 - 7.81 (m, 3H), 4.78 -4.99 (m, 2H), 3.55 - 3.59 (m, 2H), 3.35 - 3.44 (m, 2H), 2.30 - 2.47 (m, 2H), 1.85 - 2.01 (m, 6H), 1.39, 1.14, 1.04 (s, s, s, 18H) ppm. Alternatively, compound 3-1 can be obtained following the same procedure and using compounds l-4a and 2-3a instead of compounds l-5a and 2-2a as the Suzuki coupling components.
Step 2. Compound 3-1 (1.0 kg, 1.0 eq.) and IPA (7.0 L, 7.0 volume) were charged into a 20.0 L four-necked RB flask under nitrogen atm. The reaction mass was cooled to 18 - 20°C and 3.0 N HC1 in isopropyl alcohol (7.0 L, 7.0 volume) was added over a period of 90 - 120 min under nitrogen atmosphere. After stirring at 25 - 30 °C for 10 - 12 hrs under nitrogen atmosphere, HPLC analysis indicated that > 98%> compound 3-1 was consumed. Next, the reaction mass was concentrated to remove IPA under vacuum at 40 - 45 °C. The semi solid obtained was added to acetone (2.0 L, 2.0 volume) with stirring and the resulting suspension was filtered under nitrogen atmosphere. The solid was washed with acetone (2.0 L, 2.0 volume) and dried in a vacuum tray drier at 40 - 45 °C for 10 hrs to give compound 3- 2 (860 g, 94%o yield) as pale yellow solid with a purity of > 98.0%> determined by HPLC analysis. LC-MS (ESI): m/z 449.2 [M + H]+. 1H NMR (400 MHz, -DMSO): δ 10.49 - 10.59 (m, 2H), 10.10 and 9.75 (m, m, 2H), 8.60 (s, 1H), 8.31 (s, 2H), 8.15 (m, 1H), 8.13 - 8.15 (m, 2H), 7.96 - 8.09 (m, 2H), 7.82 (s, 2H), 5.08 (m, 2H), 3.39 - 3.53 (m, 4H), 2.47 - 2.54 (m, 3H), 2.37 (m, 1H), 2.14 - 2.21 (m, 2H), 2.08 (m, 2H) ppm.
Step 3. Compound 3-2 (2.2 kg, 1.0 eq.) was added to a four necked round bottom flask charged with DMF (4.4 L, 20.0 volume) under a nitrogen atmosphere. After stirring for 15 min, the mixture was added N-Moc-L-Valine (226.2 g, 3.52 eq.) in one lot at 25 - 30 °C. Next, the mixture was cooled to -20 to -15 °C, followed by adding HATU (372.9 g, 2.0 eq.) portion wise over 30 min. After stirring for 10 min, a solution of DIPEA (238.9 g, 5.0 eq.) in DMF (1.1 L, 5.0 volume) was added over 45 min. Subsequently, the reaction mass was warmed to 25 - 30 °C with stirring. After stirring for 1 hr, HPLC analysis indicated that > 99%) of compound 3-2 was consumed. The reaction mixture was poured into water (38.0 L) and the mixture was extracted with DCM (10.0 L x 3, 45.0 volume). The combined organic extracts were washed with water (10.0 L x 3, 45.0 volume) and saturated brine (10 L, 45.0 volume) and dried over anhydrous Na2S04. The solvent was removed at 40 - 45 °C under vacuum (600 mmHg) and the residue was purified by column chromatography on silica gel using DCM and MeOH as the eluent to give compound 3-3 (1.52 kg, 47% yield) as off white solid with a purity of > 97.0% determined by HPLC analysis. LC-MS (ESI): m/z 763.4 [M + H]+. 1H NMR (400 MHz, -DMSO): δ 8.60 (s, 1H), 8.29 (s, 1H), 8.20 (s, 1H), 8.09 - 8.14 (m, 2H), 7.99 - 8.05 (m, 2H), 7.86 - 7.95 (m, 3H), 7.20-7.21 (m, 2H), 5.24 - 5.33 (m, 2H), 4.06 - 4.18 (m, 4H), 3.83 (m, 2H), 3.53 (m, 6H), 2.26 - 2.55 (m, 10H), 0.85 (m, 6H), 0.78 (m, 6H) ppm. The transformation of 3-2 to 3-3 (Compound I) can be achieved via a range of conditions. One of these conditions is described below.
A reactor was charged with N-Moc-V aline (37.15 g, 0.211 mol), acetonitrile (750 mL) and DIPEA (22.5 g). The reaction mixture was agitated for 10 min and HOBT (35.3 g 0.361 mole) and EDCI (42.4 g, 0.221 mole) were added while keeping temperature < 2 °C. The reaction mixture was agitated for 30 min and DIPEA (22.5 g) and compound 3-2 (48.0 g, 0.092 mole) was added slowly to reactor over 30 min to keep temperature < 3 °C. The reaction mixture was agitated 4 hrs at 20 - 25 °C, and sample was submitted for reaction completion analysis by HPLC (IPC specification: < 1.0% area 3-2 remaining). At the completion of reaction as indicated by HPLC analysis, isopropyl acetate (750 mL) was added to the reactor and stirred for 10 min. The organic layer (product layer) was washed with brine (300 mL x 2) and 2% NaOH (200 mL). The organic solution was filtered through a silica gel pad to remove insoluble material. The silica gel pad was washed with isopropyl acetate and concentrated under vacuum (400 mm/Hg) to a minimum volume. The crude product was purified by column chromatography on silica gel using ethyl acetate and methanol as eluent to give compound 3-3 (38.0 g, 65%> yield) with purity of > 95 %>. LC-MS (ESI): m/z 763.4 [M + H]+.
Step 4. Compound 3-3 (132.0 g, 1.0 eq.) and ethanol (324.0 mL, 2.0 volume) were charged into a 10 L four-necked round bottom flask under nitrogen atmosphere. After stirring for 15 min, the suspension was cooled to 5 - 10 °C, to it was added 2.0 N HC1 in ethanol (190 mL, 1.5 volume) over 30 min. The resulting solution was allowed to warm to 25 - 30 °C. Acetone (3.96 L, 30.0 volume) was added over 90 min in to cause the slow precipitation. Next, the suspension was warmed to 60 °C and another batch of acetone (3.96 L, 30.0 volume) was added over 90 min. The temperature was maintained at 55 - 60 °C for 1 hr, and then allowed to cool to 25 - 30 °C. After stirring at 25 - 30 °C for 8 - 10 hrs, the mixture was filtered. The solid was washed with acetone (660.0 mL, 5.0 volume) and dried in a vacuum tray drier at 50 - 55 °C for 16 hrs to give the di-HCl salt of compound 3-3
(compound I) (101 g, 71% yield) as pale yellow solid with a purity of > 96.6% determined by HPLC analysis.
Preparation of N-Moc-L-Valine
N-Moc-L-Valine is available for purchase but can also be made. Moc-L-Valine was prepared by dissolving 1.0 eq of L-valine hydrochloride in 2-methyltetrahydrofuran (2- MeTHF) /water containing sodium hydroxide and sodium carbonate, and then treating with 1.0 eq of methyl chloroformate at 0 - 5°C for 6 hr. The reaction mixture was diluted with 2- MeTHF, acidified with HC1, and the organic layer was washed with water. The 2-MeTHF solution is concentrated and the compound is precipitated with n-heptane. The solid was rinsed with 2-MeTHF/ n-heptane and dried in vacuo to give N-Moc-L-Valine in 68% yield. Crystallization of Compound I to Yield Form A
Compound I Salt Formation and Crystallization, Example 1
Ethanol (3.19 L, 1.0 volume, 200 proof) was charged to the 230-L glass lined reactor under nitrogen atmosphere. Free base form of compound 3-3 (3.19 kg, 4.18 mol) was added to the flask with stirring, stir continued for an additional 20 to 30 min. To the thick solution of 3-3 in ethanol was added slowly 2.6 N HC1 in ethanol (3.19 L, 1.0 volume) to the above mass at 20 - 25 °C under nitrogen atmosphere. The entire mass was stirred for 20 min at rt, and then heated to 45 - 50 °C. Acetone (128.0 L, 40.0 volume) was added to the above reaction mass at 45 - 50 °C over a period of 3-4 hrs before it was cooled to ~25 °C and stirred for ~15 hrs. The precipitated solid was collected by filtration and washed with acetone (6.4 L x 2, 4.0 volume), suck dried for 1 hr and further dried in vacuum tray drier at 40 - 45 °C for 12 hrs. Yield: 2.5 kg (71.0% yield), purity by HPLC: 97.70%, XRPD: amorphous.
Isopropyl alcohol (7.5 L, 3.0 volume) was charged to a 50.0 L glass reactor protected under a nitrogen atmosphere. The amorphous di-HCl salt of 3-3 (2.5 kg) was added to the above reactor with stirring. The entire mass was heated to 60 - 65 °C to give a clear solution. Stir continued at 65 ± 2 °C for ~15 hrs, solid formation started during this time. The heating temperature was lowered to ~50 °C over a period of 3 hrs, methyl tertiary butyl ether (12.5 L, 5.0 volume) was added to the above mass slowly over a period of ~3 hrs with gentle agitation. The above reaction mass was further cooled to 25 - 30 °C over 2 - 3 hrs. The solid was collected by filtration, washed with 10.0% isopropyl alcohol in methyl tertiary butyl ether (6.25 L, 2.5 volume), suck dried for 1 hr and further dried in a tray drier at 45 - 50 °C under vacuum (600 mm/Hg) for 70 - 80 hrs. Yield: 2.13 kg (85.0% recovery, 61.0% yield based on the input of compound free base 3-3), purity by HPLC: 97.9%.
FIG. 1 : 1H NMR (500 MHz, -DMSO): δ 15.6 (bs, 2H), 14.7 (bs, 2H), 8.58 (s, 1H), 8.35 (s, 1H), 8.25 (s, 1H), 8.18 (d, J= 8.7 Hz, 1H), 8.13 (s, 1H), 8.06 (d, J= 8.6 Hz, 1H), 8.04 (s, 1H), 8.00 (s, 1H), 7.98 (d, J= 8.7 Hz, 1H), 7.91 (d, J= 8.6 Hz, 1H), 7.36 (d, J = 8.6 Hz, 1H), 7.33 (d, J= 8.6 Hz, 2H), 5.31 (m, 1H), 5.26 (m, 1H), 4.16 (d, J= 7.7 Hz, 1H), 4.04 (m, 2H), 3.87 (m, 2H), 3.55 (s, 6H), 2.42 (m, 2H), 2.22-2.26 (m, 4H), 2.07-2.14 (m, 4H), 0.86 (d, J= 2.6 Hz, 3H), 0.84 (d, J= 2.6 Hz, 3H), 0.78 (d, J= 2.2 Hz, 3H), 0.77 (d, J= 2.2 Hz, 3H), 3.06 (s, OMe of MTBE), 1.09 (s, t-Bu of MTBE), 1.03 (d, 2Me of IP A) ppm.
FIG. 2: 13C NMR (500 MHz, /-DMSO): δ 171.6, 171.5, 157.4, 156.1, 150.0, 138.2, 138.0, 133.5, 132.5, 131.3, 129.8, 129.4, 128.0, 127.0, 126.4, 125.6, 125.3, 124.4, 124.2, 115.8, 115.0, 112.5, 58.37, 58.26, 54.03, 53.34, 52.00 (2 carbons), 47.71 (2 carbons), 31.52, 31.47, 29.42 (2 carbons), 25.94, 25.44, 20.13, 20.07, 18.37, 18.36 ppm.
FIG. 3: FT-IR (KBr pellet): 3379.0, 2963.4, 2602.1, 1728.4, 1600.0, 1523.4, 1439.7, 1420.6, 1233.2, 1193.4, 1100.9, 1027.3 cm"1.
Elemental Analysis: Anal. Calcd for C42H52C12N806: C, 60.35; H, 6.27; N, 13.41; CI, 8.48. Found C, 58.63; H, 6.42; N, 12.65, CI, 8.2.
FIG. 1 is a representative 1H NMR spectrum of Compound I Form A.
FIG. 2 is a representative 13C NMR spectrum of Compound I Form A.
FIG. 3 is a representative FT-IR spectrum of Compound I Form A.
References:
1. Lalezari, J. P.; et. al. PPI-668, a potent new pan-genotypic HCV NS5A inhibitor: phase 1 efficacy and safety. Hepatology 2012, 56, 1065A-1066A.
1. Lalezari, J. P.; et. al. PPI-668, a potent new pan-genotypic HCV NS5A inhibitor: phase 1 efficacy and safety. Hepatology 2012, 56, 1065A-1066A.
- ClinicalTrials.govA Study of the Efficacy and Safety of PPI-668 (NS5A Inhibitor) Plus Sofosbuvir, With or Without Ribavirin, in Patients With Chronic Hepatitis C Genotype-4. NCT02371408(retrieved on 24-03-2015)
3. ClinicalTrials.gov Study of PPI-668, BI 207127 and Faldaprevir, With and Without Ribavirin, in the Treatment of Chronic Hepatitis C. NCT01859962 (retrieved on 15-09-2015)
4. Lalezari, J.; et. al. High rate of sustained virologic response in patients with hcv genotype-1a infection: a phase 2 trial of faldaprevir, deleobuvir and ppi-668, with and without ribavirin. EASL-The International Liver Congress 2014- 49th Annual Meeting of the European Association for the Study of the Liver London, United Kingdom April 9-13 (article here)
| US20070185175 * | 27 Jul 2006 | 9 Aug 2007 | Bristol-Myers Squibb Company | Benzothiazole and azabenzothiazole compounds useful as kinase inhibitors |
| US20080050336 * | 8 Aug 2007 | 28 Feb 2008 | Bristol-Myers Squibb Company | Hepatitis C Virus Inhibitors |
| WO2012087976A2 * | 19 Dec 2011 | 28 Jun 2012 | Intermune, Inc. | Novel inhibitors of hepatitis c virus replication |
| WO2013123092A1 * | 13 Feb 2013 | 22 Aug 2013 | Presidio Pharmaceuticals, Inc. | Solid forms comprising inhibitors of hcv ns5a, compositions thereof, and uses therewith |
| WO2013158776A1 * | 17 Apr 2013 | 24 Oct 2013 | Gilead Sciences, Inc. | Compounds and methods for antiviral treatment |
| US8765731 | 16 Nov 2012 | 1 Jul 2014 | Vertex Pharmaceuticals Incorporated | Benzimidazole analogues for the treatment or prevention of flavivirus infections |
| US8779156 | 24 Sep 2012 | 15 Jul 2014 | Vertex Pharmaceuticals Incorporated | Analogues for the treatment or prevention of flavivirus infections |
| US8809330 | 1 Nov 2013 | 19 Aug 2014 | Gilead Sciences, Inc. | Pyrazolo[1,5-A]pyrimidines for antiviral treatment |
| US8946238 | 20 Dec 2012 | 3 Feb 2015 | Gilead Sciences, Inc. | Pyrazolo[1,5-A]pyrimidines as antiviral agents |
| US8980878 | 17 Apr 2013 | 17 Mar 2015 | Gilead Sciences, Inc. | Compounds and methods for antiviral treatment |
| US20110274648 * | 4 Nov 2010 | 10 Nov 2011 | Bristol-Myers Squibb Company | Hepatitis C Virus Inhibitors |
////////////Phase III, Hepatitis C, RAVIDASVIR, PPI-668, BI 238630

























No comments:
Post a Comment