
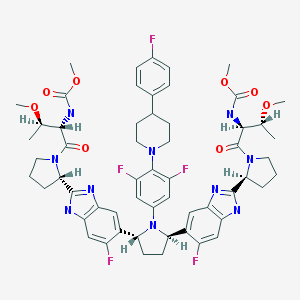
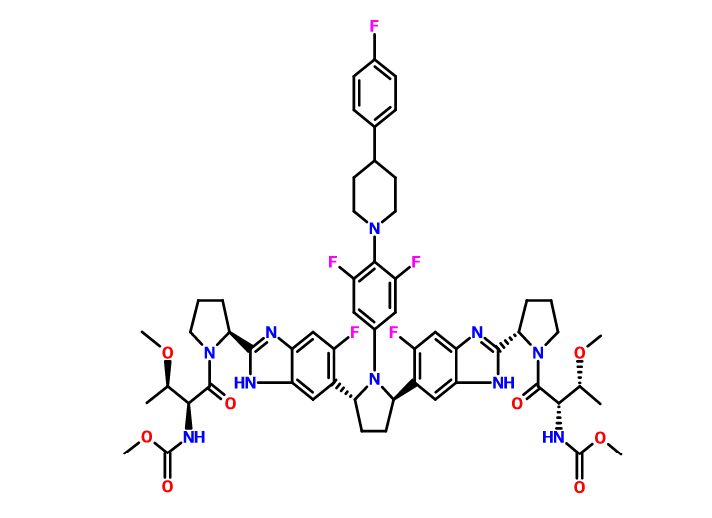
Pibrentasvir
ABT-530, Pibrentasvir, A 1325912.0
Dimethyl N,N'-([(2R,5R)-1-{3,5-difluoro-4-[4-(4-fluorophenyl)piperidin-1-yl]phenyl}pyrrolidine-2,5-diyl]bis{(6-fluoro-1H-benzimidazole-5,2-diyl)[(2S)-pyrrolidine-2,1-diyl][(2S,3R)-3-methoxy-1-oxobutane-1,2-diyl]})biscarbamate
Methyl {(2S,3R)-1-[(2S)-2-{5-[(2R,5R)-1-{3,5-difluoro-4-[4-(4-fluorophenyl)piperidin-1-yl]phenyl}-5-(6-fluoro-2-{(2S)-1-[N-(methoxycarbonyl)-O-methyl-L-threonyl]pyrrolidin-2-yl}-1H-benzimidazol-5-yl)pyrrolidin-2-yl]-6-fluoro-1H-benzimidazol-2-yl}pyrrolidin-1-yl]-3-methoxy-1-oxobutan-2-yl}carbamate
Dimethyl N,N'-(((2R,5R)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)pyrrolidine-2,5-diyl)bis((6-fluoro-1H-benzimidazole-5,2-diyl)((2S)-pyrrolidine-2,1-diyl)((2S,3R)-3-methoxy-1-oxobutane-1,2-diyl)))biscarbamate
Methyl ((2S,3R)-1-((2S)-2-(5-((2R,5R)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)-5-(6-fluoro-2-((2S)-1-(N-(methoxycarbonyl)-O-methyl-L-threonyl)pyrrolidin-2-yl)-1H-benzimidazol-5-yl)pyrrolidin-2-yl)-6-fluoro-1H-benzimidazol-2-yl)pyrrolidin-1-yl)-3-methoxy-1-oxobutan-2-yl)carbamate
Phase III
Abbott Laboratories INNOVATOR
A protease inhibitor potentially for the treatment of HCV infection.Hepatitis C virus NS 5 protein inhibitors

CAS No. 1353900-92-1
| MF | C57H65F5N10O8 |
|---|
MW 1113.1925 MW
- CLINICAL https://clinicaltrials.gov/search/intervention=A-1325912.0%20OR%20ABT-530%20OR%20Pibrentasvir
 Pibrentasvir
Pibrentasvir
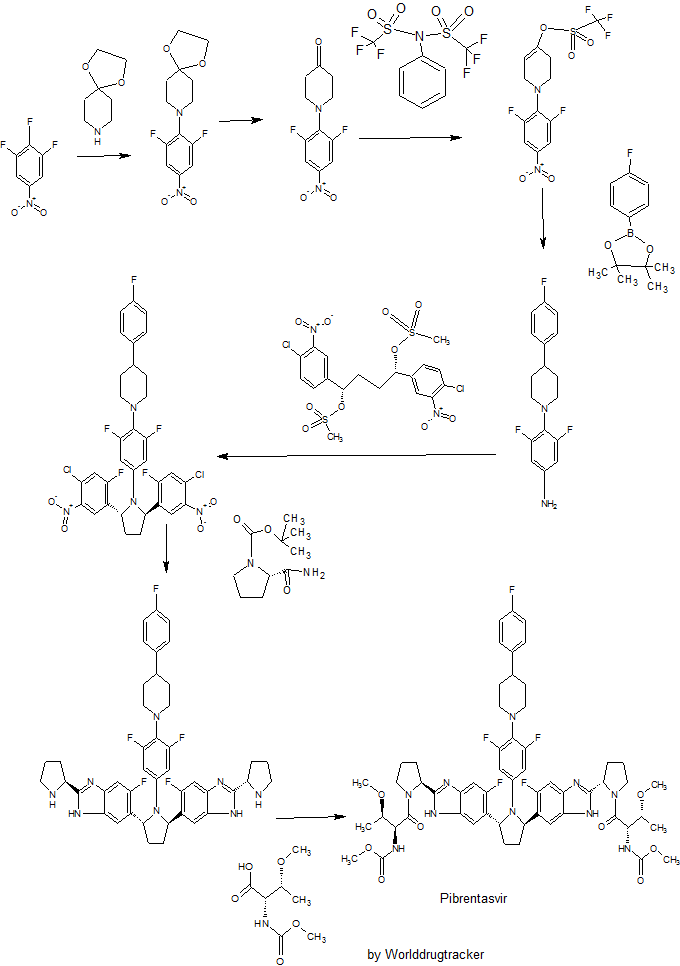
WO 2012051361
Example 3.52 methyl {(2S,3R)-l-[(2S)-2-{5-[(2R,5R)-l-{3,5-difluoro-4-[4-(4- fluorophenyl)piperidin-l-yl]phenyl}-5-(6-fluoro-2-{(2.S)-l-[A^-(methoxycarbonyl)-0-methyl-L- threonyl]pyiTolidin-2-yl}-l f-benzimidazol-5-yl)pyiTolidin-2-yl]-6-fluoro-l f-benzimidaz yl}pyrrolidin-l-yl]-3-methoxy-l-oxobutan-2-yl}carbamatelH NMR (400 MHz, DMSO) δ 12.36 - 12.06 (m, 2H), 7.41 (dd, J = 11.2, 6.3, 1H), 7.34 (dd, J = 10.4, 4.8, 1H), 7.30 - 7.20 (m, 3H), 7.17 - 6.98 (m, 5H), 5.98 - 5.82 (m, 2H), 5.65 - 5.47 (m, 2H), 5.17 - 5.06 (m, 2H), 4.25 (dd, J = 15.6, 8.1, 2H), 3.88 - 3.74 (m, 3H), 3.53 (d, J = 1.3, 6H), 3.49 - 3.38 (m, 2H), 3.31 (d, 1H), 3.25 (d, J = 3.7, 1H), 3.13 (d, J = 1.3, 3H), 3.03 (d, J = 2.3, 3H), 3.00 - 2.84 (m, 3H), 2.60 - 2.53 (m, J = 2.5, 2H), 2.26 - 1.55 (m, 14H), 1.28 - 1.13 (m, 1H), 1.10 - 0.88 (m, 6H). MS (ESI; M+H) m/z = 1113.4.
PATENT
The present invention features crystalline polymorphs of methyl {(2S,3R)-1- [(2S)-2-{5-[(2R,5R)-l-{3,5-difluoro-4 4-(4-fluorophenyl)piperidin-l-yl]phenyl}-5-(6-fluoro-2-{(2S)- 1 -[N-(methoxycarbonyl)-0-methyl-L-threonyl]pyrrolidin-2-yl} - 1 H-benzimidazol-5-yl)pyrrolidin- -yl] -6-fluoro- 1 H-benzimidazol-2-yl} pyrrolidin- 1 -yl] -3 -methoxy- 1 -oxobutan-2-
yl} carbamate 
, herein "Compound I"). Compound I is a potent HCV NS5A inhibitor and is described in U.S. Patent Application Publication No. 2012/0004196, which is incorporated herein by reference in its entirety.

, herein "Compound I"). Compound I is a potent HCV NS5A inhibitor and is described in U.S. Patent Application Publication No. 2012/0004196, which is incorporated herein by reference in its entirety.
//////////1353900-92-1, PHASE 3, ABT-530, Pibrentasvir, ABT 530, A 1325912.0
C[C@H]([C@@H](C(=O)N1CCC[C@H]1c2[nH]c3cc(c(cc3n2)[C@H]4CC[C@@H](N4c5cc(c(c(c5)F)N6CCC(CC6)c7ccc(cc7)F)F)c8cc9c(cc8F)[nH]c(n9)[C@@H]1CCCN1C(=O)[C@H]([C@@H](C)OC)NC(=O)OC)F)NC(=O)OC)OC
C[C@H]([C@@H](C(=O)

No comments:
Post a Comment