Dapiprazole
CAS 72822-12-9
HCL SALT 72822-13-0
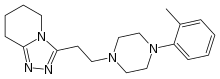
| 5,6,7,8-Tetrahydro-3-(2-(4-(O-tolyl)-1-piperazinyl)ethyl)-S-triazolo(4,3-a)pyridine |
Dapiprazole (Rev-Eyes) is an alpha blocker. It is used to reverse mydriasis after eye examination.[1]
Used in the treatment of iatrogenically induced mydriasis produced by adrenergic (phenylephrine) or parasympatholytic (tropicamide) agents used in certain eye examinations.
Dapiprazole is an alpha-adrenergic blocking agent. It produces miosis by blocking the alpha-adrenergic receptors on the dilator muscle of the iris. Dapiprazole produces no significant action on ciliary muscle contraction and thus, there are no changes in the depth of the anterior chamber of the thickness of the lens. It does not alter the IOP either in normal eyes or in eyes with elevated IOP. The rate of pupillary constriction may be slightly slower in clients with brown irises than in clients with blue or green irises.
Dapiprazole acts through blocking the alpha1-adrenergic receptors in smooth muscle. It produces miosis through an effect on the dilator muscle of the iris and does not have any significant activity on ciliary muscle contraction and, therefore does not induce a significant change in the anterior chamber depth or the thickness of the lens.
Oral LD50 is 1189-2100 mg/kg in mice, rats and rabbits.

Brief background information
| Salt | ATC | formula | MM | CASE |
|---|---|---|---|---|
| - | N05AX S01EX02 | C19H27N5 | 325.46 g / mol | 72822-12-9 |
| monogïdroxlorïd | N05AX S01EX02 | C19H27N5 · HCl | 361.92 g / mol | 72822-13-0 |
Application
- antipsihoticheskoe means
- in the treatment of glaucoma
Classes substance
- Piperazinы
- 1,2,4-triazolo [4,3-a] piridinы
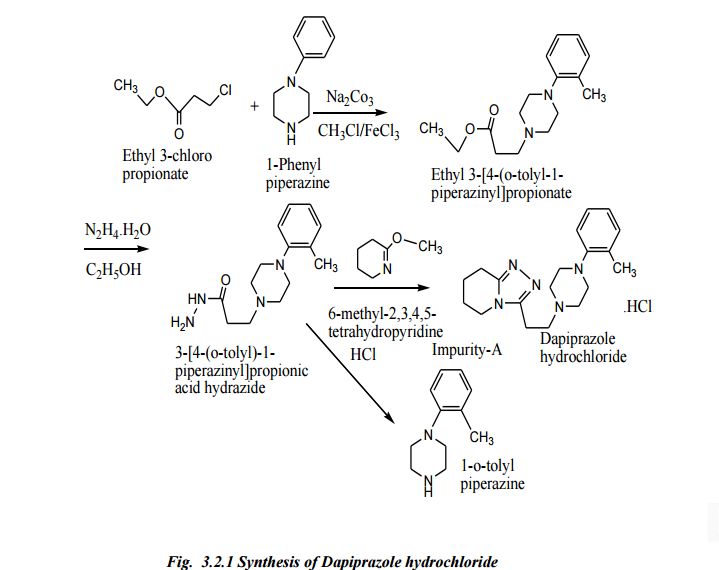
Synthesis
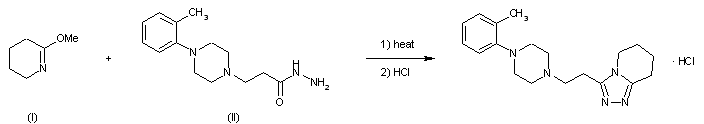 | |
| Scheme illustration:By cyclization of O-methylvalerolactam (I) with 3-(4-o-tolyl-1-piperazinyl) propionic acid hydrazide (II) in refluxing xylene, followed by a treatment with ethanolic HCl. |
FR 2423221; GB 2020269; JP 54157576; NL 7902489; US 4252721
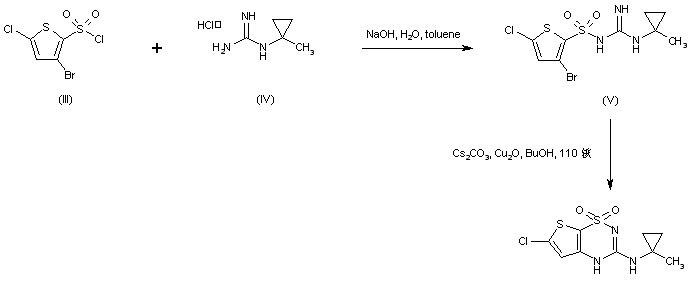
Acylation of (1-methylcyclopropyl)guanidine (IV) with 3-bromo-5-chlorothiophene-2-sulfonyl chloride (III) under Schotten-Baumann conditions afforded the sulfonyl guanidine (V). This was cyclized to the desired thienothiadiazine upon treatment with Cs2CO3 and Cu2O in boiling butanol.
In a different method, (1-methylcyclopropyl)guanidine (I) is acylated by 3-bromo-5-chlorothiophene-2-sulfonyl chloride (II) to produce the sulfonyl guanidine (III). Intramolecular cyclization of (III) in the presence of Cu2O and Cs2CO3 leads to the title thienothiadiazine derivative. Similarly, acylation of guanidine (I) with 3,5-dichlorothiophene-2-sulfonyl chloride (IV) provides sulfonyl guanidine (V), which is then cyclized in the presence of Cu2O and Cs2CO3.
In an alternative method, sulfonylation of N-isopropylguanidine (V) with 2,5-dichlorothiophene-3-sulfonyl chloride (IV) produced the sulfonyl guanidine (VI). This was then cyclized to the title compound by treatment with copper bronze and potassium carbonate in boiling DMF........WO 0102410
Trade names
| country | Tradename | Manufacturer |
|---|---|---|
| Germany | Remidrial | winegrower |
| Italy | Glamidolo | Angelini, 1987 |
| Ukraine | no | no |
Formulations
- eyedrops 50 mg / 10 ml (5%) (hydrochloride)
References
- DE 2 915 318 (Angelini; appl. 14.4.1979; I-prior. 18.4.1978).
- US 4 307 095 (Angelini; 22.12.1981; prior. 29.3.1979, 29.8.1980; I-prior. 18.4.1978).
- US 4 307 096 (Angelini; 22.12.1981; prior. 29.3.1979, 29.8.1980; I-prior. 18.4.1978).
- US 4 325 952 (Angelini; 20.4.1982; prior. 29.3.1979, 29.8.1980; I-prior. 18.4.1978).
- BE 877 161 (Angelini; appl. 21.6.1979).
References
- Doughty, Michael J.; Lyle, William M. (May 1992). "A Review of the Clinical Pharmacokinetics of Pilocarpine, Moxisylyte (Thymoxamine), and Dapiprazole in the Reversal of Diagnostic Pupillary Dilation". Optometry & Vision Science 69 (5).
- US 4 307 096 (Angelini; 22.12.1981; prior. 29.3.1979, 29.8.1980; I-prior. 18.4.1978).
- US 4 325 952 (Angelini; 20.4.1982; prior. 29.3.1979, 29.8.1980; I-prior. 18.4.1978).
- BE 877 161 (Angelini; appl. 21.6.1979).
- DE 2 915 318 (Angelini; appl. 14.4.1979; I-prior. 18.4.1978).
- US 4 307 095 (Angelini; 22.12.1981; prior. 29.3.1979, 29.8.1980; I-prior. 18.4.1978).
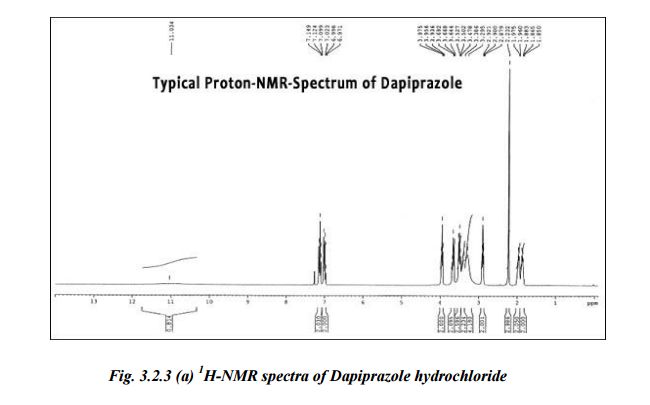
Structural formula
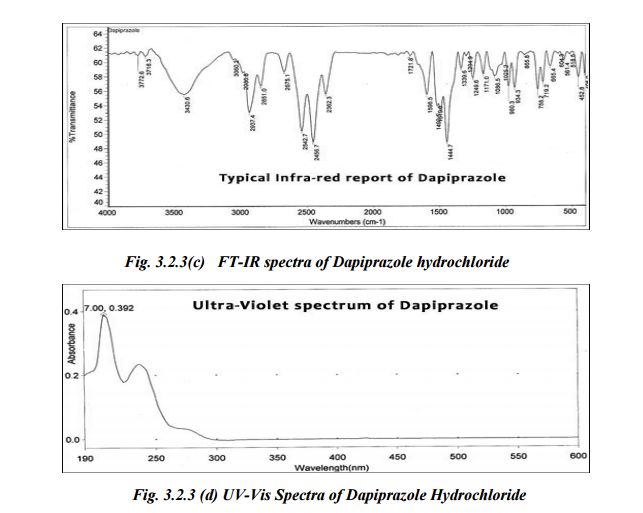
UV- Spectrum
IR - spectrum
References
- UV and IR Spectra. H.-W. Dibbern, R.M. Muller, E. Wirbitzki, 2002 ECV
- NIST/EPA/NIH Mass Spectral Library 2008
- Handbook of Organic Compounds. NIR, IR, Raman, and UV-Vis Spectra Featuring Polymers and Surfactants, Jr., Jerry Workman. Academic Press, 2000.
- Handbook of ultraviolet and visible absorption spectra of organic compounds, K. Hirayama. Plenum Press Data Division, 1967.
 | |
| Systematic (IUPAC) name | |
|---|---|
| 3-{2-[4-(2-methylphenyl)piperazin-1-yl]ethyl}-5,6,7,8- tetrahydro-[1,2,4]triazolo[4,5-a]pyridine | |
| Clinical data | |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a601043 |
| Pregnancy category |
|
| Routes of administration | Topical (eye drops) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Negligible when administered topically |
| Identifiers | |
| CAS Number | 72822-12-9 |
| ATC code | S01EX02 (WHO) |
| PubChem | CID 3033538 |
| IUPHAR/BPS | 7155 |
| DrugBank | DB00298 |
| ChemSpider | 2298190 |
| UNII | 5RNZ8GJO7K |
| KEGG | D07775 |
| ChEBI | CHEBI:51066 |
| ChEMBL | CHEMBL1201216 |
| Chemical data | |
| Formula | C19H27N5 |
| Molar mass | 325.451 g/mol |
//////Дапипразол , Dapiprazole, AF-2139, Remydrial, Rev-Eyes, Reversil, Glamidolo
n1nc(n2c1CCCC2)CCN4CCN(c3ccccc3C)CC4











No comments:
Post a Comment