BTI-320 (formerly PAZ320)
PAZ 320
Non-insulin dependent diabetes
Alpha-glucosidase inhibitor; Hydrolase inhibitor; Sucrose alpha-glucosidase inhibitor
Composition of chemically purified (fractionation) soluble mannan polysaccharides from legume's seeds
BTI-320 is in phase II clinical development at Boston Therapeutics for the treatment of type 2 diabetes in combination with oral agents or insulin, and also for the treatment of high-risk patients with pre-diabetes. A chewable tablet formulation is being developed. The product is already available as dietary supplement.
| Company | Boston Therapeutics Inc. |
| Description | Chewable polysaccharide that inhibits alpha glucosidase |
| Molecular Target | |
| Mechanism of Action | Alpha glucosidase inhibitor |
| Therapeutic Modality | Macromolecule: Polysaccharide |
| Latest Stage of Development | Phase II |
| Standard Indication | Diabetes |
| Indication Details | Treat Type II diabetes |


PATENT
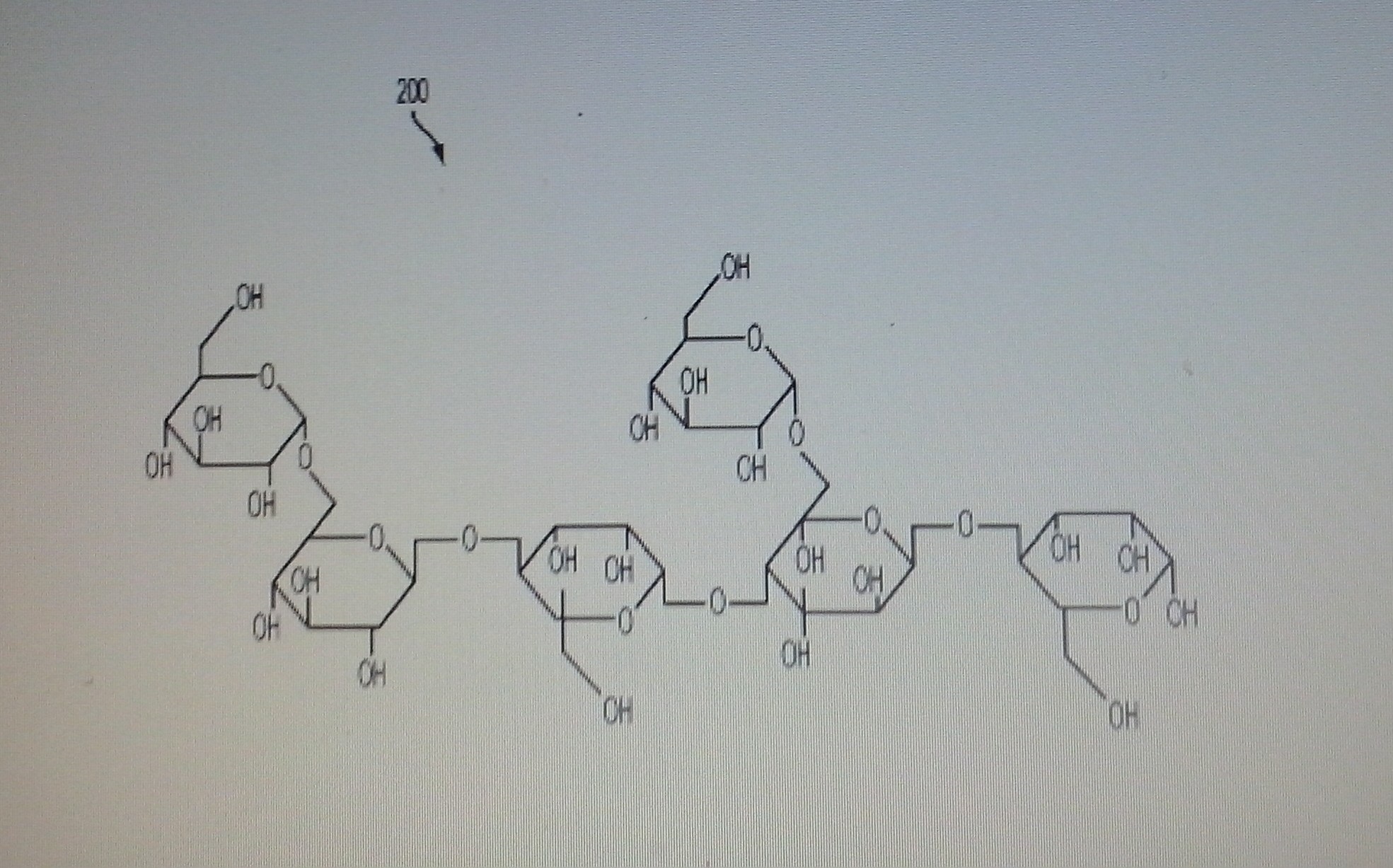
A composition of chemically purified soluble mannans from legumes' seeds (e.g. Ceratonia siliqua, Cæsalpinia spinosa Trigonelle foenum-graecum, and Cyamopsis tetragonolobus) and their use in the assembly of palatable dietary supplements is disclosed herein. The fractionation process provides high-quality physiologically soluble, chemically modified and purified homogeneous size polysaccharide fibers, devoid of natural impurities, for example proteins, alkaloids, glycoalkaloids, and/or environmental impurities including heavy metals, agricultural residues and microbial toxins. This process provides hypoallergenic dietary fibers devoid of any potential allergens, cytotoxins, and gastrointestinal toxins. A sequential process for assembly of the soluble fibers with plurality of molecular weights to create a time controlled dissolution of the functional high and low molecular weight fibers for improving solubility and palatability with improved dietary performance in the oral and gastro-intestinal system is also disclosed herein.
Fig. 1 illustrates a block flow diagram of an embodiment of a method for recovering purified mannan polysaccharides;
Fig. 2 illustrates a chemical structure of a mannan polysaccharide;
Fig. 3 illustrates a block flow diagram of an embodiment of a method for recovering high molecular weight (HMW) purified mannan polysaccharides;
Fig. 4 illustrates a block flow diagram of an embodiment of a method for recovering low molecular weight (LMW) purified mannan polysaccharides;
REFERENCES
https://clinicaltrials.gov/show/NCT02060916
https://clinicaltrials.gov/show/NCT02358668
BTI-320, a nonsystemic novel drug to control glucose uptake into the bloodstream, functions as a competitive inhibitor of sugar hydrolyzing enzymes
75th Annu Meet Sci Sess Am Diabetes Assoc (ADA) (June 5-9, Boston) 2015, Abst 974-P
75th Annu Meet Sci Sess Am Diabetes Assoc (ADA) (June 5-9, Boston) 2015, Abst 974-P
Boston Therapeutics' Hong Kong Affiliate Advance Pharmaceutical's BTI-320 Clinical Trial Reaches Mid-Point by Enrolling 30 Patients at the Chinese University of Hong Kong
Boston Therapeutics Press Release 2015, July 08
Boston Therapeutics Press Release 2015, July 08
Insight into the molecular mechanism of action of BTI320, a non-systemic novel drug to control serum glucose levels in individuals with diabetes50th Annu Meet Eur Assoc Study Diabetes (EASD) (September 15-19, Vienna) 2014, Abst 545
////BTI-320, PAZ320, PHASE 2, BTI 320, PAZ 320, Macromolecule, Polysaccharide, Non-insulin dependent diabetes, Alpha-glucosidase inhibitor, Hydrolase inhibitor, Sucrose alpha-glucosidase inhibitor, phase II clinical development, Boston Therapeutics, Soluble mannan polysaccharides
Composition of chemically purified (fractionation) soluble mannan polysaccharides from legume's seeds
POLYMER OF BELOW
CAS 9036-88-8, 51395-96-1
| refractive index : | 78.5 ° (C=1.4, H2O) |
Ailes;MANNAN;K-41K1;D-Mannan;NSC 174478;NSC 174479;NSC 174481;NSC 307194;NSC 174477;NSC 174473
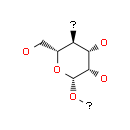
| Chemical name: | 1,6-Anhydro-β-D-mannopyranose |
| Synonyms: | 1,6-Anhydro-D-mannose; 1,6-Anhydromannose; Mannosan; NSC 226600; |
| CAS Number: | 14168-65-1 |
| Possible CAS #: | NA |
| Molecular form.: | C₆H₁₀O₅ |
| Appearance: | White to Pale Beige Solid |
| Melting Point: | 182-184°C |
| Mol. Weight: | 162.14 |
Summary:
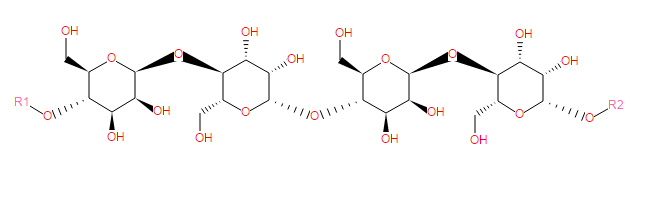
Mannans are major constitutents of hemicelluloses in plant tissue and are polymers composed of β(1→4)-linked mannose and glucose residues. Some contain galactopyranosyl side chains (see a galactomannan).
Mannans are major constitutents of hemicelluloses in plant tissue and are polymers composed of β(1→4)-linked mannose and glucose residues. Some contain galactopyranosyl side chains (see a galactomannan).
Slightly galactosylated mannans (4% galactose), considered as linear β(1→4)-D-mannans, have been isolated from the seed endosperm of vegetable ivory nut ( Phytelephas macrocarpa) and date ( Phoenix dactylifera) .
Glycan icon:

Child Classes: a 1,6-α-D-mannan backbone (0), a galactoglucomannan (0), a galactomannan (0), a glucomannan (0), a mannan oligosaccharide (1)
SMILES: C(O)C4(C(O[R1])C(O)C(O)C(OC3(C(O)C(O)C(OC2(C(O)C(O)C(OC1(C(O)C(O)C(O[R2])OC(CO)1))OC(CO)2))OC(CO)3))O4)
CAS:9036-88-8,
No comments:
Post a Comment