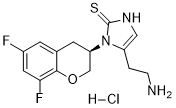
Etamicastat HCl salt
CAS: 677773-32-9 (HCl salt)
CAS 760173-05-5 (free base).
Chemical Formula: C14H16ClF2N3OS
Molecular Weight: 347.8088
Synonym: BIA 5-453; BIA5-453; BIA-5-453; Etamicastat
IUPAC/Chemical Name: (R)-5-(2-aminoethyl)-1-(6,8-difluorochroman-3-yl)-1,3-dihydro-2H-imidazole-2-thione hydrochloride
5-(2-Aminoethyl)-1-((3R)-6,8-difluoro-3,4-dihydro-2H-chromen-3-yl)-1,3-dihydro-2h-imidazole-2-thione
R)-5-(2-aminoethyl)-1-(6,8-difluorochroman-3-yl)-1,3-dihydroimidazole-2-thione hydrochloride,PHASE 2, Treatment of Heart Failure Therapy, Hypertension
Bial-Portela and Ca, S.A

is a novel peripherally selective dopamine β-hydroxylase (DBH) inhibitor being developed by Bial-Portela and Ca, S.A. for treatment of hypertension and congestive heart failure.(1) The compound was shown to be well tolerated in healthy volunteers.
Etamicastat, also known as BIA 5-453, is a potent, reversible, peripherally selective dopamine β-hydroxylase inhibitor (DBH inhibitor). Chronic dopamine ß-hydroxylase inhibition with etamicastat effectively decreases blood pressure, although does not prevent the development of hypertension in the spontaneously hypertensive rat.

aReagents and conditions: a) Boc2O, EtOH, rt, 2 h; b) TBDMS-Cl, Et3N, DMAP, DCM, rt, 18 h; c) Dess–Martin periodinane, DCM, rt, 1 h; d) 2, KSCN, AcOH, EtOAc, reflux, 7 h; e) 2 N HCl, EtOAc, rt, 2 h.
Paper
Development of the Asymmetric Hydrogenation Step for Multikilogram Production of Etamicastat
Laboratory of Chemistry, Department of Research & Development, BIAL, 4745-457 S. Mamede do Coronado, Portugal
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.6b00041
Publication Date (Web): March 21, 2016
Copyright © 2016 American Chemical Society

The asymmetric hydrogenation of methyl (6,8-difluoro-2H-chromen-3-yl)carbamate
is a key step in the manufacturing route to etamicastat. A development
of this step including the ruthenium or rhodium catalyst screening and
the influence of the catalyst preparation (isolated, preformed in
solution or in situ), solvent, temperature, pressure, additive, and
concentration on the performance of the given ligand was discussed.
Scale-up experiments for the best catalysts under optimized conditions
were described.
PAPER
Synthesis
and biological evaluation of novel, peripherally selective chromanyl
imidazolethione-based inhibitors of dopamine beta-hydroxylase
J Med Chem 2006, 49(3): 1191
J Med Chem 2006, 49(3): 1191
PATENT
in the processes .
(J?) -5- (2-Aminoethyl) -1- (6, 8-difluorochroman-3-yl) -1, 3-dihydroimidazole-2 -thione hydrochloride (the compound of formula 1, below) is a potent, non-toxic and peripherally selective inhibitor of ϋβΗ, which can be used for treatment of certain cardiovascular disorders. Compound 1 is disclosed in WO2004/033447 , along with processes for its preparation.

1
The process disclosed in WO2004/033447 involves the reaction of ( R) - 6 , 8 -difluorochroman-3 -ylamine hydrochloride (the structure of ( R) -6, 8-difluorochroman-3 -ylamine is shown below as compound QA) , [4 - ( tert-butyldimethylsilanyloxy) -3 -oxobutyl] carbamic acid tert-butyl ester and potassium thiocyanate .

QA
(R) -6 , 8-difluorochroman- 3 -ylamine (compound QA) is a key intermediate in the synthesis of compound 1. The stereochemistry at the carbon atom to which the amine is attached gives rise to the stereochemistry of compound 1, so it is advantageous that compound QA is present in as pure enantiomeric form as possible. In other words, the (R) -enantiomer of compound QA should be in predominance, with little or no (S) enantiomer present. Thus, the process for preparing compound QA will advantageously produce compound QA with as high enantiomeric excess (ee) as possible.
Advantageous processes for preparing, for example, the compound of formula QA have now been found. In one aspect, the processes involve a biotransformation step. In another aspect, the processes involve chemical transformation. The processes may also be employed in the preparation of similar precursors useful in the production of other peripherally-selective inhibitors of dopamine -β -hydroxylase .
WO2008/136695 discloses a compound of formula YA, its (R) or (S) enantiomer, a mixture of its (R) and (S) enantiomers, or pharmaceutically acceptable salts thereof.

YA
The (R) -enantiomer of the compound of formula YA has been found to be a potent dopamines-hydroxylase inhibitor having high potency and significantly reduced brain access.
As disclosed in WO2008/136695 , the compound of formula YA may be prepared by reacting the compound of formula 1 with benzaldehyde under reductive alkylation conditions. In particular, (R) -5- (2 -aminoethyl ) -1- (6 , 8-difluorochroman-3 -yl) - 1 , 3 -dihydroimidazole-2 -thione and benzaldehyde may be reacted in the presence of a solvent or mixture of solvents, and a reducing agent such as sodium cyanoborohydride or sodium triacetoxyborohydride .
(J?) -5- (2-Aminoethyl) -1- (6, 8-difluorochroman-3-yl) -1, 3-dihydroimidazole-2 -thione hydrochloride (the compound of formula 1, below) is a potent, non-toxic and peripherally selective inhibitor of ϋβΗ, which can be used for treatment of certain cardiovascular disorders. Compound 1 is disclosed in WO2004/033447 , along with processes for its preparation.

1
The process disclosed in WO2004/033447 involves the reaction of ( R) - 6 , 8 -difluorochroman-3 -ylamine hydrochloride (the structure of ( R) -6, 8-difluorochroman-3 -ylamine is shown below as compound QA) , [4 - ( tert-butyldimethylsilanyloxy) -3 -oxobutyl] carbamic acid tert-butyl ester and potassium thiocyanate .

QA
(R) -6 , 8-difluorochroman- 3 -ylamine (compound QA) is a key intermediate in the synthesis of compound 1. The stereochemistry at the carbon atom to which the amine is attached gives rise to the stereochemistry of compound 1, so it is advantageous that compound QA is present in as pure enantiomeric form as possible. In other words, the (R) -enantiomer of compound QA should be in predominance, with little or no (S) enantiomer present. Thus, the process for preparing compound QA will advantageously produce compound QA with as high enantiomeric excess (ee) as possible.
Advantageous processes for preparing, for example, the compound of formula QA have now been found. In one aspect, the processes involve a biotransformation step. In another aspect, the processes involve chemical transformation. The processes may also be employed in the preparation of similar precursors useful in the production of other peripherally-selective inhibitors of dopamine -β -hydroxylase .
WO2008/136695 discloses a compound of formula YA, its (R) or (S) enantiomer, a mixture of its (R) and (S) enantiomers, or pharmaceutically acceptable salts thereof.

YA
The (R) -enantiomer of the compound of formula YA has been found to be a potent dopamines-hydroxylase inhibitor having high potency and significantly reduced brain access.
As disclosed in WO2008/136695 , the compound of formula YA may be prepared by reacting the compound of formula 1 with benzaldehyde under reductive alkylation conditions. In particular, (R) -5- (2 -aminoethyl ) -1- (6 , 8-difluorochroman-3 -yl) - 1 , 3 -dihydroimidazole-2 -thione and benzaldehyde may be reacted in the presence of a solvent or mixture of solvents, and a reducing agent such as sodium cyanoborohydride or sodium triacetoxyborohydride .
The compound of formula W may be prepared using a process as disclosed herein from the nitro chromene compound M.
The compound of formula WA may also be prepared using a process comprising bromination of 2 , 4 -difluorophenol to give bromophenol, alkylation of bromophenol with 4 -chloro-3 -oxo butanoate to give ketone followed by cyclization and decarboxylation to produce compound WA.

WA
The compound of formula WA may also be prepared using a process comprising bromination of 2 , 4 -difluorophenol to give bromophenol, alkylation of bromophenol with 4 -chloro-3 -oxo butanoate to give ketone followed by cyclization and decarboxylation to produce compound WA.

WA
According
to an aspect of the present invention, there is provided the following 2
-part synthetic route from the starting material 2 , 4 -difluorophenol
to (R) -5- (2 -aminoethyl ) -1- (6 , 8-difluorochroman-3 -yl) -1 , 3
-dihydroimidazole-2 - thione
hydrochloride :
Part (1)


Preferred reagents and conditions:
a) HMTA, CF3COOH, 115°C, 18 hours
b) CH2CHCN, DABCO, DMF, water, 70°C, 16 hours
c) H2S04, AcOH, 100°C, 1 hour
d) NaClO, NaOH, MeOH, 25°C, 24 hours
e) (R) -C3 -TunePhosRu (acac) 2 S/C 3000, 30 bar H2, MeOH, 80°C, 20 hours
f) Water, 2-propanol, reflux to 20°C
g) 40% KOH, MeOH, reflux, 24 hours
h) L-tartaric acid, ethanol, water, RT, 1 hour
Part (2)



Preferred reagents and conditions
a') methyl vinyl ketone, t-BuONa, EtOAc, EtOH, 40-50°C, 2-3 hours
Br2, MeOH, 20-25°C, 5 hours
water, reflux, 1 hour
KOH, AcOH, reflux, 1 hour
HCl, water, 2-propanol, 75 °C, 4 hours
KSCN, AcOH, 100°C, 2-4 hours
NaHC03, water, EtOH
NaBH4, 2-propanol, THF, water, 20-25°C, 16 hours
HCl, 2-propanol, water, reflux, 1-2 hours
The ( R ) -5- (2-Aminoethyl) -1- (6, 8-difluorochroman-3 -yl) -1,3-dihydroimidazole-2 - thione hydrochloride
hydrochloride :
Part (1)


Preferred reagents and conditions:
a) HMTA, CF3COOH, 115°C, 18 hours
b) CH2CHCN, DABCO, DMF, water, 70°C, 16 hours
c) H2S04, AcOH, 100°C, 1 hour
d) NaClO, NaOH, MeOH, 25°C, 24 hours
e) (R) -C3 -TunePhosRu (acac) 2 S/C 3000, 30 bar H2, MeOH, 80°C, 20 hours
f) Water, 2-propanol, reflux to 20°C
g) 40% KOH, MeOH, reflux, 24 hours
h) L-tartaric acid, ethanol, water, RT, 1 hour
Part (2)


Preferred reagents and conditions
a') methyl vinyl ketone, t-BuONa, EtOAc, EtOH, 40-50°C, 2-3 hours
Br2, MeOH, 20-25°C, 5 hours
water, reflux, 1 hour
KOH, AcOH, reflux, 1 hour
HCl, water, 2-propanol, 75 °C, 4 hours
KSCN, AcOH, 100°C, 2-4 hours
NaHC03, water, EtOH
NaBH4, 2-propanol, THF, water, 20-25°C, 16 hours
HCl, 2-propanol, water, reflux, 1-2 hours
The ( R ) -5- (2-Aminoethyl) -1- (6, 8-difluorochroman-3 -yl) -1,3-dihydroimidazole-2 - thione hydrochloride
EXAMPLES
Example 1
Nitro chromene synthesis

To 3 , 5-difluoro-2-hydroxybenzaldehyde (lOg, 63mmol, leq) , di-n-butylamine (4.1g, 32mmol, 0.5eq) , phtalic anhydride (18.7g, 126mmol, 2eq) in toluene (500mL) was added nitroethanol (5.75g, 63mmol, leq) . The round bottomed flask fitted with a dean stark apparatus was refluxed for 18h. The mixture was cooled and nitroethanol (5.75g, 63mmol, leq) was added. The resulting reaction mixture was then reflux for 12h. After cooling, the solution was evaporated down to approximately 150mL and purified over silica gel (eluent ethyl acetate : hexane 1:1) this gave several fractions that contained only the product by TLC, these was evaporated under reduced pressure to yield 1.8g which was 100% pure by HPLC aera. Several more fractions were collected containing a mixture of product and starting material. These were combined and washed with 2% NaOH solution (2x50mL) to remove starting material. The organic layer was washed with water (50mL) , dried over sodium sulfate and evaporated under reduced pressure to give 2.49g of brown solid ( 100% pure by HPLC aera) . More fractions were collected. These were combined, washed with 2% NaOH solution (3xl00mL) , water (lOOmL) and dried over sodium sulfate. This was then filtered and evaporated down in vacuum to yield 6.14g of a brown solid which was 91.3% pure by HPLC aera. 6 , 8 -difluoro-3 -nitro-2H-chromene (9.90g, 73.4%) was obtained as a brown solid.
Example 2
Nitro chromene synthesis with column purification
To a solution of isobenzofuran-1 , 3 -dione (4,68 g, 31,6 mmol) , 3 , 5-difluoro-2 -hydroxybenzaldehyde (2,5 g, 15,81 mmol) in Toluene (25 ml) was added 2 -nitroethanol (2,88 g, 31,6 mmol). The resulting mixture was heated to reflux overnight (Dean stark) .
The reaction conversion was checked by TLC (eluent PE/EtOAc 9:1) . A yellow spot was observed and corresponds to the expected product .
Reaction was cooled to room temperature and a plug of silica gel was performed. A pale brown solid (3.9g) was obtained. """H-NMR showed presence of product and starting material. The solid was dissolved in diethylether and the organic layer was washed with aqueous sodium carbonate, dried over Na2S04, filtered and concentrated under reduced pressure. A pale brown solid (1.7g,) was obtained. The 1H-NMR was indicated no starting material but still polymer from nitroethanol and residue of phtalic anhydride. A second silica plug (eluent: PE/EtOAc 95:5) was done. A pale yellow solid (1.5g) was obtained. 1H-NMR of solid showed only product and polymer. The solid was recrystallized from methanol/water . A pale yellow solid (1.05g, 31.2%) was obtained.
Example 3
Nitro chromene synthesis without column purification
To a solution of isobenzofuran- 1 , 3 -dione (18,74 g, 127 mmol) , 3 , 5-difluoro-2 -hydroxybenzaldehyde (10 g, 63,3 mmol) in Toluene (100 ml) was added 2 -nitroethanol (6,86 ml, 95 mmol) . The resulting mixture was heated to reflux for 24h (Dean stark) .
The reaction conversion was checked by HPLC and by 1H-NMR. Only 50% conversion was obtained.
The reaction mixture was cooled to room temperature and diluted with DCM (lOOmL) and 1M NaOH solution (200mL) .
The biphasic system was stirred for 30 minutes and then separated (very difficult to see phase separation) . The aqueous layer was washed with DCM (50mL) and the combined organic layers were washed twice with water (2x50ml) , dried over sodium sulfate. The filtered organic layer was concentrated under reduced pressure. To the residue was added methanol (50mL) . The methanol was then removed by distillation under reduced pressure. A brown solution precipitated when most of the methanol was removed. More methanol was added and more solid crushed out then few drops of water was added to increase the product precipitation. The brown slurry was stirred for 30 minutes and filtered. The brown solid was washed with methanol/water (1:9, 5mL) and dried in a vacuum oven at 40°C for 12h.6, 8-difluoro-3 -nitro-2H-chroraene (4,9 g, 22,99 mmol,) was obtained as brown solid in 36.3% yield.
HPLC showed a purity of 98% and 1H-NMR confirmed the structure and purity around 95%
Example 4
Reduction of nitro chromene to nitro-alkane (racemic mixture)

To a suspension of 6 , 8 -difluoro-3 -nitro-2H-chromene (213mg, 0,999 mmol) and silica (0,8 g, 0,999 mmol) in a mixture of CHC13 (10 ml) and IPA (3,4 ml) at 0°C was added portion wise sodium borohydride (95 mg, 2,498 mmol). The resulting mixture was stirred at 0°C for 45 minutes. Reaction conversion was checked by HPLC. 1 mL of acetic acid was added at 0°C and the resulting mixture was stirred for 30 minutes at room temperature. The slurry was filtered and the silica was washed with DCM. The filtrate was diluted with ethyl acetate and water and the biphasic system was separated. The aqueous layer was back extracted with ethyl acetate. The combined organic layers were washed with brine, dried over MgS04, filtered and concentrated under reduced pressure.
6 , 8-difluoro-3 -nitrochroman (196mg, 0,911 mmol, 91 % yield) was obtained as a pale yellow oil.
Example 5
Preparation of 6 , 8 -difluorochroman-3 -one from nitro chromene

A solution of 6, 8-difluoro-3 -nitro-2H-chromene (lOOmg, 0,469 mmol) in acetic acid (0.5 ml) is added slowly to a stirred slurry of iron (262 mg, 4,69 mmol) in acetic acid (1 ml) at 60.deg. C. The reaction mixture is stirred at 60. °C for 2 hour then allowed to cool to room temperature and stirred overnight. The reaction mixture is poured onto ice-water (30 ml) and filtered through Celite. The solid was wash with dichloromethane (DCM) (50 ml) . The organic portion is separated and washed with water (2 x 30 ml) and brine (30 ml) , dried over MgS04, filtered and concentrated in vacuo to give a brown oil. 6,8-difluorochroman-3 -one (75 mg, 0,407 mmol, 87 % yield) was obtained as a brown oil.
Example 6
Preparation of 6 , 8-difluorochroman-3 -one from methyl 6,8-difluoro-2H-chromen-3 -yl-carbamate

Methanol (1000m ml) was added to a slurry of methyl fluoro-2H-chromen-3 -yl -carbamate (250 g, 1.037 mol) hydrogen chloride 6N (2000 ml, 12 mol) at room temperature. The resulting mixture was reflux and stirred for 2 hours. Reaction monitored by HPLC.
Reaction was not complete but was stopped in order to avoid degradation of the product. The yellow solution was cooled to room temperature. A slurry (two type of solid) was observed and diluted with diethyl ether (300mL) . The resulting slurry was stirred at 5°C for 1 hour then filtered. The yellow solid was washed with water. The resulting wet yellow solid was suspended in diethylether (400mL) and petroleum ether (PE) (400mL) was added. Slight yellow solid was stirred at room temperature overnight, filtered and washed with PE (300mL) , dried in a vacuum oven at 30 °C for 4h. The wet sample was checked by NMR. No starting material was detected. A pale yellow solid (72.5g, solid 1) was obtained. The mother liquors were concentrated to dryness. A yellow solid was obtained, suspended in diethyl ether and PE. The slurry was then stirred for 4 hours, filtered, washed with PE . A dark yellow solid (4.5g, solid 2) was obtained. Solid 1 (2g) was diluted in DCM and washed with water (pH =6). The organic layer was then dried over Na2S04, filtered, concentrated to dryness. A crystalline pale yellow solid (1.9g, solid 3) was obtained. NMR showed the same purity for solid 3 as for solid 1. The remaining part of solid 1 was then diluted in DCM. The resulting organic layer was washed with water, dried over Na2S04, filtered and then concentrated to dryness. Slight yellow crystalline solid (68.5g, solid 4) was obtained. NMR confirmed high quality material.
Loss on Drying (LOD) : 1.03% .
Example 7
Biotransformation: Transaminases

Codexis transaminases ATA-025, ATA-251 and ATA-P2-A07 recognized 6 , 8 -difluorochroman-3 -one as the substrate and produced the corresponding 6 , 8 -difluorochroman-3 -amine .
Example 1
Nitro chromene synthesis

To 3 , 5-difluoro-2-hydroxybenzaldehyde (lOg, 63mmol, leq) , di-n-butylamine (4.1g, 32mmol, 0.5eq) , phtalic anhydride (18.7g, 126mmol, 2eq) in toluene (500mL) was added nitroethanol (5.75g, 63mmol, leq) . The round bottomed flask fitted with a dean stark apparatus was refluxed for 18h. The mixture was cooled and nitroethanol (5.75g, 63mmol, leq) was added. The resulting reaction mixture was then reflux for 12h. After cooling, the solution was evaporated down to approximately 150mL and purified over silica gel (eluent ethyl acetate : hexane 1:1) this gave several fractions that contained only the product by TLC, these was evaporated under reduced pressure to yield 1.8g which was 100% pure by HPLC aera. Several more fractions were collected containing a mixture of product and starting material. These were combined and washed with 2% NaOH solution (2x50mL) to remove starting material. The organic layer was washed with water (50mL) , dried over sodium sulfate and evaporated under reduced pressure to give 2.49g of brown solid ( 100% pure by HPLC aera) . More fractions were collected. These were combined, washed with 2% NaOH solution (3xl00mL) , water (lOOmL) and dried over sodium sulfate. This was then filtered and evaporated down in vacuum to yield 6.14g of a brown solid which was 91.3% pure by HPLC aera. 6 , 8 -difluoro-3 -nitro-2H-chromene (9.90g, 73.4%) was obtained as a brown solid.
Example 2
Nitro chromene synthesis with column purification
To a solution of isobenzofuran-1 , 3 -dione (4,68 g, 31,6 mmol) , 3 , 5-difluoro-2 -hydroxybenzaldehyde (2,5 g, 15,81 mmol) in Toluene (25 ml) was added 2 -nitroethanol (2,88 g, 31,6 mmol). The resulting mixture was heated to reflux overnight (Dean stark) .
The reaction conversion was checked by TLC (eluent PE/EtOAc 9:1) . A yellow spot was observed and corresponds to the expected product .
Reaction was cooled to room temperature and a plug of silica gel was performed. A pale brown solid (3.9g) was obtained. """H-NMR showed presence of product and starting material. The solid was dissolved in diethylether and the organic layer was washed with aqueous sodium carbonate, dried over Na2S04, filtered and concentrated under reduced pressure. A pale brown solid (1.7g,) was obtained. The 1H-NMR was indicated no starting material but still polymer from nitroethanol and residue of phtalic anhydride. A second silica plug (eluent: PE/EtOAc 95:5) was done. A pale yellow solid (1.5g) was obtained. 1H-NMR of solid showed only product and polymer. The solid was recrystallized from methanol/water . A pale yellow solid (1.05g, 31.2%) was obtained.
Example 3
Nitro chromene synthesis without column purification
To a solution of isobenzofuran- 1 , 3 -dione (18,74 g, 127 mmol) , 3 , 5-difluoro-2 -hydroxybenzaldehyde (10 g, 63,3 mmol) in Toluene (100 ml) was added 2 -nitroethanol (6,86 ml, 95 mmol) . The resulting mixture was heated to reflux for 24h (Dean stark) .
The reaction conversion was checked by HPLC and by 1H-NMR. Only 50% conversion was obtained.
The reaction mixture was cooled to room temperature and diluted with DCM (lOOmL) and 1M NaOH solution (200mL) .
The biphasic system was stirred for 30 minutes and then separated (very difficult to see phase separation) . The aqueous layer was washed with DCM (50mL) and the combined organic layers were washed twice with water (2x50ml) , dried over sodium sulfate. The filtered organic layer was concentrated under reduced pressure. To the residue was added methanol (50mL) . The methanol was then removed by distillation under reduced pressure. A brown solution precipitated when most of the methanol was removed. More methanol was added and more solid crushed out then few drops of water was added to increase the product precipitation. The brown slurry was stirred for 30 minutes and filtered. The brown solid was washed with methanol/water (1:9, 5mL) and dried in a vacuum oven at 40°C for 12h.6, 8-difluoro-3 -nitro-2H-chroraene (4,9 g, 22,99 mmol,) was obtained as brown solid in 36.3% yield.
HPLC showed a purity of 98% and 1H-NMR confirmed the structure and purity around 95%
Example 4
Reduction of nitro chromene to nitro-alkane (racemic mixture)

To a suspension of 6 , 8 -difluoro-3 -nitro-2H-chromene (213mg, 0,999 mmol) and silica (0,8 g, 0,999 mmol) in a mixture of CHC13 (10 ml) and IPA (3,4 ml) at 0°C was added portion wise sodium borohydride (95 mg, 2,498 mmol). The resulting mixture was stirred at 0°C for 45 minutes. Reaction conversion was checked by HPLC. 1 mL of acetic acid was added at 0°C and the resulting mixture was stirred for 30 minutes at room temperature. The slurry was filtered and the silica was washed with DCM. The filtrate was diluted with ethyl acetate and water and the biphasic system was separated. The aqueous layer was back extracted with ethyl acetate. The combined organic layers were washed with brine, dried over MgS04, filtered and concentrated under reduced pressure.
6 , 8-difluoro-3 -nitrochroman (196mg, 0,911 mmol, 91 % yield) was obtained as a pale yellow oil.
Example 5
Preparation of 6 , 8 -difluorochroman-3 -one from nitro chromene

A solution of 6, 8-difluoro-3 -nitro-2H-chromene (lOOmg, 0,469 mmol) in acetic acid (0.5 ml) is added slowly to a stirred slurry of iron (262 mg, 4,69 mmol) in acetic acid (1 ml) at 60.deg. C. The reaction mixture is stirred at 60. °C for 2 hour then allowed to cool to room temperature and stirred overnight. The reaction mixture is poured onto ice-water (30 ml) and filtered through Celite. The solid was wash with dichloromethane (DCM) (50 ml) . The organic portion is separated and washed with water (2 x 30 ml) and brine (30 ml) , dried over MgS04, filtered and concentrated in vacuo to give a brown oil. 6,8-difluorochroman-3 -one (75 mg, 0,407 mmol, 87 % yield) was obtained as a brown oil.
Example 6
Preparation of 6 , 8-difluorochroman-3 -one from methyl 6,8-difluoro-2H-chromen-3 -yl-carbamate

Methanol (1000m ml) was added to a slurry of methyl fluoro-2H-chromen-3 -yl -carbamate (250 g, 1.037 mol) hydrogen chloride 6N (2000 ml, 12 mol) at room temperature. The resulting mixture was reflux and stirred for 2 hours. Reaction monitored by HPLC.
Reaction was not complete but was stopped in order to avoid degradation of the product. The yellow solution was cooled to room temperature. A slurry (two type of solid) was observed and diluted with diethyl ether (300mL) . The resulting slurry was stirred at 5°C for 1 hour then filtered. The yellow solid was washed with water. The resulting wet yellow solid was suspended in diethylether (400mL) and petroleum ether (PE) (400mL) was added. Slight yellow solid was stirred at room temperature overnight, filtered and washed with PE (300mL) , dried in a vacuum oven at 30 °C for 4h. The wet sample was checked by NMR. No starting material was detected. A pale yellow solid (72.5g, solid 1) was obtained. The mother liquors were concentrated to dryness. A yellow solid was obtained, suspended in diethyl ether and PE. The slurry was then stirred for 4 hours, filtered, washed with PE . A dark yellow solid (4.5g, solid 2) was obtained. Solid 1 (2g) was diluted in DCM and washed with water (pH =6). The organic layer was then dried over Na2S04, filtered, concentrated to dryness. A crystalline pale yellow solid (1.9g, solid 3) was obtained. NMR showed the same purity for solid 3 as for solid 1. The remaining part of solid 1 was then diluted in DCM. The resulting organic layer was washed with water, dried over Na2S04, filtered and then concentrated to dryness. Slight yellow crystalline solid (68.5g, solid 4) was obtained. NMR confirmed high quality material.
Loss on Drying (LOD) : 1.03% .
Example 7
Biotransformation: Transaminases

Codexis transaminases ATA-025, ATA-251 and ATA-P2-A07 recognized 6 , 8 -difluorochroman-3 -one as the substrate and produced the corresponding 6 , 8 -difluorochroman-3 -amine .
PATENT
WO 2004033447
WO 2008094056
WO 2008143540
WO 2009064210
References
1: Igreja B, Wright LC, Soares-da-Silva P. Sustained high blood pressure reduction with etamicastat, a peripheral selective dopamine β-hydroxylase inhibitor. J Am Soc Hypertens. 2015 Dec 19. pii: S1933-1711(15)00838-4. doi: 10.1016/j.jash.2015.12.011. [Epub ahead of print] PubMed PMID: 26803288.2: Loureiro AI, Bonifácio MJ, Fernandes-Lopes C, Pires N, Igreja B, Wright LC, Soares-da-Silva P. Role of P-glycoprotein and permeability upon the brain distribution and pharmacodynamics of etamicastat: a comparison with nepicastat. Xenobiotica. 2015;45(9):828-39. doi: 10.3109/00498254.2015.1018985. Epub 2015 Jun 10. PubMed PMID: 25915108.
3: Loureiro AI, Soares-da-Silva P. Distribution and pharmacokinetics of etamicastat and its N-acetylated metabolite (BIA 5-961) in dog and monkey. Xenobiotica. 2015;45(10):903-11. doi: 10.3109/00498254.2015.1024780. Epub 2015 Apr 14. PubMed PMID: 25869244.
4: Pires NM, Igreja B, Moura E, Wright LC, Serrão MP, Soares-da-Silva P. Blood pressure decrease in spontaneously hypertensive rats folowing renal denervation or dopamine β-hydroxylase inhibition with etamicastat. Hypertens Res. 2015 Sep;38(9):605-12. doi: 10.1038/hr.2015.50. Epub 2015 Apr 9. PubMed PMID: 25854989.
5: Bonifácio MJ, Sousa F, Neves M, Palma N, Igreja B, Pires NM, Wright LC, Soares-da-Silva P. Characterization of the interaction of the novel antihypertensive etamicastat with human dopamine-β-hydroxylase: comparison with nepicastat. Eur J Pharmacol. 2015 Mar 15;751:50-8. doi: 10.1016/j.ejphar.2015.01.034. Epub 2015 Jan 29. PubMed PMID: 25641750.
6: Pires NM, Loureiro AI, Igreja B, Lacroix P, Soares-da-Silva P. Cardiovascular safety pharmacology profile of etamicastat, a novel peripheral selective dopamine-β-hydroxylase inhibitor. Eur J Pharmacol. 2015 Mar 5;750:98-107. doi: 10.1016/j.ejphar.2015.01.035. Epub 2015 Jan 30. PubMed PMID: 25641747.
7: Igreja B, Pires NM, Bonifácio MJ, Loureiro AI, Fernandes-Lopes C, Wright LC, Soares-da-Silva P. Blood pressure-decreasing effect of etamicastat alone and in combination with antihypertensive drugs in the spontaneously hypertensive rat. Hypertens Res. 2015 Jan;38(1):30-8. doi: 10.1038/hr.2014.143. Epub 2014 Oct 9. PubMed PMID: 25298210.
8: Loureiro AI, Bonifácio MJ, Fernandes-Lopes C, Igreja B, Wright LC, Soares-da-Silva P. Etamicastat, a new dopamine-ß-hydroxylase inhibitor, pharmacodynamics and metabolism in rat. Eur J Pharmacol. 2014 Oct 5;740:285-94. doi: 10.1016/j.ejphar.2014.07.027. Epub 2014 Jul 21. PubMed PMID: 25058908.
9: Almeida L, Nunes T, Costa R, Rocha JF, Vaz-da-Silva M, Soares-da-Silva P. Etamicastat, a novel dopamine β-hydroxylase inhibitor: tolerability, pharmacokinetics, and pharmacodynamics in patients with hypertension. Clin Ther. 2013 Dec;35(12):1983-96. doi: 10.1016/j.clinthera.2013.10.012. Epub 2013 Dec 2. PubMed PMID: 24296323.
10: Loureiro AI, Rocha JF, Fernandes-Lopes C, Nunes T, Wright LC, Almeida L, Soares-da-Silva P. Human disposition, metabolism and excretion of etamicastat, a reversible, peripherally selective dopamine β-hydroxylase inhibitor. Br J Clin Pharmacol. 2014 Jun;77(6):1017-26. doi: 10.1111/bcp.12274. PubMed PMID: 24168152; PubMed Central PMCID: PMC4093927.
11: Loureiro AI, Fernandes-Lopes C, Bonifácio MJ, Wright LC, Soares-da-Silva P. N-acetylation of etamicastat, a reversible dopamine-β-hydroxylase inhibitor. Drug Metab Dispos. 2013 Dec;41(12):2081-6. doi: 10.1124/dmd.113.053736. Epub 2013 Sep 6. PubMed PMID: 24013186.
12: Nunes T, Rocha JF, Vaz-da-Silva M, Falcão A, Almeida L, Soares-da-Silva P. Pharmacokinetics and tolerability of etamicastat following single and repeated administration in elderly versus young healthy male subjects: an open-label, single-center, parallel-group study. Clin Ther. 2011 Jun;33(6):776-91. doi: 10.1016/j.clinthera.2011.05.048. PubMed PMID: 21704242.
13: Vaz-da-Silva M, Nunes T, Rocha JF, Falcão A, Almeida L, Soares-da-Silva P. Effect of food on the pharmacokinetic profile of etamicastat (BIA 5-453). Drugs R D. 2011;11(2):127-36. doi: 10.2165/11587080-000000000-00000. PubMed PMID: 21548660; PubMed Central PMCID: PMC3585837.
14: Rocha JF, Vaz-Da-Silva M, Nunes T, Igreja B, Loureiro AI, Bonifácio MJ, Wright LC, Falcão A, Almeida L, Soares-Da-Silva P. Single-dose tolerability, pharmacokinetics, and pharmacodynamics of etamicastat (BIA 5-453), a new dopamine β-hydroxylase inhibitor, in healthy subjects. J Clin Pharmacol. 2012 Feb;52(2):156-70. doi: 10.1177/0091270010390805. PubMed PMID: 21343348.
15: Nunes T, Rocha JF, Vaz-da-Silva M, Igreja B, Wright LC, Falcão A, Almeida L, Soares-da-Silva P. Safety, tolerability, and pharmacokinetics of etamicastat, a novel dopamine-β-hydroxylase inhibitor, in a rising multiple-dose study in young healthy subjects. Drugs R D. 2010;10(4):225-42. doi: 10.2165/11586310-000000000-00000. PubMed PMID: 21171669; PubMed Central PMCID: PMC3585840.
16: Beliaev A, Learmonth DA, Soares-da-Silva P. Synthesis and biological evaluation of novel, peripherally selective chromanyl imidazolethione-based inhibitors of dopamine beta-hydroxylase. J Med Chem. 2006 Feb 9;49(3):1191-7. PubMed PMID: 16451083.
PATENT CITATIONS
| Cited Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| WO1995007284A1 * | Aug 29, 1994 | Mar 16, 1995 | Smithkline Beecham Plc | Phosphinic acid derivatives with anti-hyper glycemic and/or anti-obesity activity |
| WO2006044293A2 * | Oct 11, 2005 | Apr 27, 2006 | Pharmacopeia Drug Discovery, Inc. | Bicyclic compounds as selective melanin concentrating hormone receptor antagonists for the treatment of obesity and related disorders |
| WO2012007548A1 * | Jul 14, 2011 | Jan 19, 2012 | Dsm Ip Assets B.V. | (r)-selective amination |
| WO2013002660A2 * | Jun 29, 2012 | Jan 3, 2013 | BIAL - PORTELA & Cª, S.A. | Process |
| GR1005093B * | Title not available |
| Reference | ||
|---|---|---|
| 1 | * | AL NEIRABEYEH M. ET AL.: "Methoxy and hydroxy derivatives of 3,4-dihydro-3-(di-n-propylamino)-2H-1-benzopyrans: new synthesis and dopaminergic activity", EUROPEAN JOURNAL OF MEDICINAL CHEMISTRY, vol. 26, no. 5, 1991, EDITIONS SCIENTIFIQUE ELSEVIER, PARIS; FR, pages 497 - 504, XP023870436, ISSN: 0223-5234, DOI: 10.1016/0223-5234(91)90145-D |
| 2 | * | BELIAEV, A. ET AL.: "Process Research for Multikilogram Production of Etamicastat: A Novel Dopamine ß-Hydroxylase Inhibitor", ORGANIC PROCESS RESEARCH & DEVELOPMENT, no. 16, 2012, American Chemical Society, Washington; US, pages 704 - 709, XP002731798, DOI: 10.1021/op300012d |
| 3 | * | BOYE, S. ET AL.: "N,N-Disubstituted aminomethyl benzofuran derivatives: synthesis and preliminary binding evaluation", BIOORGANIC & MEDICINAL CHEMISTRY, no. 7, 1999, ELSEVIER SCIENCE LTD; GB, pages 335 - 341, XP002731795, ISSN: 0968-0896, DOI: 10.1016/S0968-0896(98)00239-9 |
| 4 | * | COMOY, C. ET AL.: "3-Amino-3,4-dihydro-2H-1-benzopyran Derivatives as 5-HT1A Receptor Ligandsand Potential Anxiolytic Agents. 2. Synthesis and QuantitativeStructure-Activity Relationship Studies of Spiro[pyrrolidine- andpiperidine-2,3'(2'H)-benzopyrans]", JOURNAL OF MEDICINAL CHEMISTRY., vol. 39, no. 21, 1996, AMERICAN CHEMICAL SOCIETY. WASHINGTON; US, pages 4285 - 4298, XP002731797, ISSN: 0022-2623, DOI: 10.1021/JM950861W |
| 5 | * | SHIN, C. ET AL.: "Total Synthesis of Bistratamide G, a Metabolite of the PhilippinesAscidian Lissoclinum bistratum, from Dehydrotripeptides", CHEMISTRY LETTERS, vol. 33, no. 6, 2004, Chemical Society of Japan, Tokyo; JP, pages 664 - 665, XP002731799, ISSN: 0366-7022, DOI: 10.1246/cl.2004.664 |
| 6 | * | VASSE, J. L. ET AL.: "New efficient conditions for the reduction with NADH models", SYNLETT, October 1998 (1998-10-01), THIEME INTERNATIONAL, STUTTGART; DE, pages 1144 - 1146, XP002731796, ISSN: 0936-5214, DOI: 10.1055/s-1998-1876 |
| 7 | * | XIAO, G.-Q. ET AL.: "3-Nitro-2H-chromenes as a New Class of Inhibitors against Thioredoxin Reductase and Proliferation of Cancer Cells", ARCHIV DER PHARMAZIE, no. 345, 2012, VCH VERLAGSGESELLSCHAFT MBH, WEINHEIM; DE, pages 767 - 770, XP002731794, ISSN: 0365-6233, DOI: 10.1002/ardp.201200121 |
SMILES Code: FC1=CC(F)=C(OC[C@H](N2C(CCN)=CNC2=S)C3)C3=C1.[H]Cl
c1c(cc(c2c1C[C@H](CO2)n3c(c[nH]c3=S)CCN)F)F
No comments:
Post a Comment